New Data Presented Today at the American Society for Experimental Neurotherapeutics (ASENT) 2020 Meeting
DEVON, Pa., March 03, 2020 (GLOBE NEWSWIRE) -- Zynerba Pharmaceuticals, Inc. (NASDAQ:ZYNE), the leader in innovative pharmaceutically-produced transdermal cannabinoid therapies for rare and near-rare neuropsychiatric disorders, is presenting data this week further describing the baseline characteristics of the pediatric and adolescent patients in the fully-enrolled Phase 2 BRIGHT trial of Zygel™ (CBD transdermal gel; ZYN002) in children and adolescents with autism spectrum disorder (ASD), indicating that the trial enrolled a broad population of patients with moderate-to-severe ASD.
The poster entitled Phase 2 BRIGHT (An Exploratory Open-Label Tolerability and Efficacy Study of ZYN002 Administered as a Transdermal Gel to Children and Adolescents with Autism Spectrum Disorder) Trial: Baseline Characteristics (poster #27) is being presented at the American Society for Experimental Neurotherapeutics (ASENT) 2020 Meeting. The poster is being presented on Tuesday, March 3 from 5:00 to 6:00 PM EST and Wednesday, March 4 from 5:00 to 7:00 PM EST. These data will also be presented during the ASENT Pipeline Data Blitz session on Wednesday March 4, 2020 from 1:00 to 3:00PM EST. The meeting is being held in Bethesda, MD on March 2nd through March 5th, 2020. A copy of the poster is available on the Zynerba corporate website at http://zynerba.com/publications/.
Zynerba’s Chief Medical Officer, Joseph M. Palumbo, MD, FAPA, MACPsych, is presenting data describing the baseline characteristics of patients enrolled in the ongoing BRIGHT trial, which indicate a patient population with predominantly moderate-to-severe ASD as measured by key scales used for screening and efficacy assessment. These include the Aberrant Behavior Checklist – Community (ABC-C); the Autism Diagnostic Observation Schedule® (ADOS-2); and the Parent Rated Anxiety Scale–Autism Spectrum Disorder (PRAS-ASD).
“ASD is a complex neurodevelopmental disorder characterized by difficulties with behaviors, communication, and social interaction,” said Dr. Palumbo. “Pediatric and adolescent patients with ASD may also present with profound clinical anxiety, above the rate seen in neurotypical children, further complicating their condition and treatment regimen. Unfortunately, current ASD management options are restricted to cognitive behavioral therapy and a small number of approved pharmacologic treatments, highlighting the substantial unmet need for novel therapies in this population. We believe that we have enrolled an appropriate population of patients into our well-designed exploratory BRIGHT trial to enable a robust analysis of outcomes to help inform the design of future double-blind, placebo-controlled studies.”
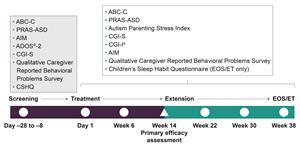
The endocannabinoid system - a key modulator of emotion and social behavior - is dysregulated in ASD, and published data suggest that cannabidiol (CBD) may provide therapeutic benefit. However, the efficacy and safety of CBD in patients with ASD have not been well established. Zynerba is undertaking the 14-week BRIGHT Phase 2 exploratory trial in children and adolescents (ages four through 17 years) with ASD as confirmed by Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) diagnostic criteria to assess the safety and efficacy of Zygel in treating ASD-related behaviors as measured by a variety of efficacy assessments which are shown in Figure 1, below. After completing dosing in the 14-week period, participants may enroll in a six-month extension trial.
Figure 1. Schedule of Screening and Efficacy Assessments is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/a15d5dd4-9ab2-497d-a8ec-7782f612ebca.
The trial protocol included certain inclusion and exclusion criteria to enrich the trial population for disease severity at baseline, as measured by the following assessments:
ABC-C
- A 58-item caregiver-rated scale measuring behaviors across 5 subscales: irritability/agitation (maximum score: 45), lethargy/social withdrawal (maximum score: 48), stereotypic behavior (maximum score: 21), hyperactivity/noncompliance (maximum score: 48), inappropriate speech (maximum score: 12);
- Each behavior is scored from 0 (“not at all a problem”) to 3 (“the problem is severe in degree”);
- Higher scores indicate greater severity of aberrant behavior.
ADOS-2
- A diagnostic tool consisting of 5 age - and verbal ability - dependent modules that assess social communication and core behaviors of ASD;
- Each item is scored by a trained test administrator from 0 (“no abnormality of type specified”) to 3 (“moderate to severe abnormality”);
- ADOS total scores are diagnostic; however, standardized comparison scores can be used to measure severity;
- Comparison scores range from 0-10, with scores of <5 indicating mild ASD, scores of 5-7 indicating moderate ASD, and scores of 8-10 indicating severe ASD.
PRAS-ASD
- A 25-item parent-rated scale assessing anxiety in ASD;
- Each item is scored from 0 (“not present”) to 3 (“very frequent and a major problem”);
- Maximum score is 75, with scores >52 indicating possible clinical anxiety.
Baseline Disease Characteristics
As seen in Table 1 below, the majority of patients had moderate or severe ASD at baseline as measured by the ADOS-2 comparison score (94%) and DSM-5 severity levels (92%). In addition, the mean ABC-C Irritability score was 30.0, and 24% of the enrolled patients had PRAS-ASD scores indicative of possible clinical anxiety, further highlighting the severity of symptoms in the enrolled patient population.
Table 1. Baseline Disease Characteristics of Patients Enrolled in BRIGHT
| Disease Characteristics | Patients in BRIGHT N=37 |
|
| ABC-C Irritability Subscale score (0-45) | ||
| n | 37 | |
| Mean (range) | 30.0 (18-43) | |
| PRAS-ASD score (0-75; >52 suggests possible clinical anxiety) | ||
| n | 37 | |
| Mean (range) | 40.9 (21-68) | |
| >52, n (%) | 9 (24.3) | |
| DSM-5 severity leveli | ||
| Level 1 (mild), n (%) | 3 (8.1) | |
| Level 2 (moderate), n (%) | 15 (40.5) | |
| Level 3 (severe), n (%) | 19 (51.4) | |
| ADOS®-2 comparison score | ||
| n | 36 | |
| Mean (range) | 7.5 (4-10) | |
| <5 (mild ASD), n (%) | 2 (5.6) | |
| 5-7 (moderate ASD), n (%) | 19 (52.8) | |
| 8-10 (severe ASD), n (%) | 15 (41.7) | |
The authors conclude that the Phase 2 BRIGHT trial has successfully enrolled a broad patient population and was enriched for disease severity to avoid floor effects on outcome measures. The baseline characteristics indicate a patient population with predominantly moderate-to-severe ASD, with a high level of clinically significant anxiety.
About Zynerba Pharmaceuticals, Inc.
Zynerba Pharmaceuticals is the leader in pharmaceutically-produced transdermal cannabinoid therapies for rare and near-rare neuropsychiatric disorders. We are committed to improving the lives of patients and their families living with severe, chronic health conditions including Fragile X syndrome, autism spectrum disorder, 22q11.2 deletion syndrome, and a heterogeneous group of rare and ultra-rare epilepsies known as developmental and epileptic encephalopathies. Learn more at www.zynerba.com and follow us on Twitter at @ZynerbaPharma.
Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from the Company’s current expectations. Management’s expectations and, therefore, any forward-looking statements in this press release could also be affected by risks and uncertainties relating to a number of other factors, including the following: the Company’s cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; the Company’s ability to obtain additional funding to support its clinical development programs; the results, cost and timing of the Company’s clinical development programs, including any delays to such clinical trials relating to enrollment or site initiation; clinical results for the Company’s product candidates may not be replicated or continue to occur in additional trials and may not otherwise support further development in a specified indication or at all; actions or advice of the U.S. Food and Drug Administration and foreign regulatory agencies may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the need for additional clinical trials; the Company’s ability to obtain and maintain regulatory approval for its product candidates, and the labeling under any such approval; the Company’s reliance on third parties to assist in conducting pre-clinical and clinical trials for its product candidates; delays, interruptions or failures in the manufacture and supply of the Company’s product candidates the Company’s ability to commercialize its product candidates; the size and growth potential of the markets for the Company’s product candidates, and the Company’s ability to service those markets; the Company’s ability to develop sales and marketing capabilities, whether alone or with potential future collaborators; the rate and degree of market acceptance of the Company’s product candidates; the Company’s expectations regarding its ability to obtain and adequately maintain sufficient intellectual property protection for its product candidates; and the timing and outcome of current and future legal proceedings. This list is not exhaustive and these and other risks are described in the Company’s periodic reports, including the annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, filed with or furnished to the Securities and Exchange Commission and available at www.sec.gov. Any forward-looking statements that the Company makes in this press release speak only as of the date of this press release. The Company assumes no obligation to update forward-looking statements whether as a result of new information, future events or otherwise, after the date of this press release.
Zynerba Contact
Will Roberts, VP Investor Relations and Corporate Communications
484.581.7489
robertsw@zynerba.com
Media contact
Molly Devlin
Evoke KYNE
215.928.2199
Molly.Devlin@evokegroup.com
_______________
i DSM-5 severity levels are based on degree of social communication impairment and behavioral flexibility. The levels indicate patients “requiring support” (level 1), “requiring substantial support” (level 2), and “requiring very substantial support” (level 3).