COVID-19 has impacted diagnostic volumes, mix and reimbursement for a variety of diagnostic providers.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20200922005762/en/
(Graphic: Business Wire)
“Six months into the COVID-19 pandemic, diagnostic labs nationwide continue to see high testing volumes. Early implementation of rapid diagnostics testing could positively impact overall testing volumes and data collection for the remainder of 2020 and into 2021, but we’re not there yet,” said Lâle White, Executive Chair and CEO at XIFIN. “Labs are making a significant effort to manage large quantities of both clinical and administrative data, from testing results to patient insurance information, claims and reimbursement – it’s a tall order. So-called ‘clean orders’ are a huge focus and critical measurement for labs in terms of profitability and the patient experience. XIFIN is committed to providing labs – so critical to the pandemic effort – with the ability to better manage their data and optimize both workflow and revenues.”
Fall Testing Volumes Expected to Rise
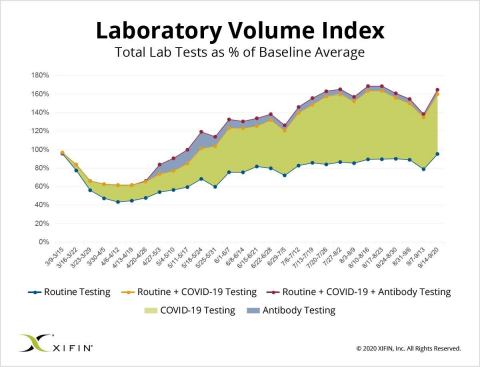
For the past six months, XIFIN has analyzed its vast stores of data to inform its Lab Volume Index, a weekly reporting of lab testing volume data across the US. This month, data shows an uptick in COVID-19 testing in early September, a seemingly direct correlation with back-to-school activity and the start of cold and flu season for many states.
This increase in testing is expected to continue throughout the fall and into the winter months. Based on current data, XIFIN experts predict labs will experience an increase in COVID-19 testing volumes of up to 15% from the August low point, as well as a possible uptick in the use of a combination flu and COVID-19 PCR test. According to the XIFIN Lab Volume Index, the overall industry has returned to approximately 95% of its baseline volume (pre-COVID) for routine testing, and for the first time, the hospital segment has routine testing that exceeds its baseline volumes.
Regional Labs Alleviate High Testing Levels and Reporting Demands
Reporting criteria from the Centers for Disease Control and Prevention (CDC) is frequently changing, making it increasingly difficult for labs to constantly update their systems and methodologies to adequately report the data being requested by the CDC. This is causing reporting delays and other obstacles at a time when labs are under extreme pressure to manage testing demands across diagnostic sectors.
Based on the activity seen within XIFIN’s lab network, the company reports that regional labs, who have smaller staffs and less resources than larger labs, are still conducting testing at two or three times their average volumes. Regional labs are securing more local employer and provider COVID-19 testing contracts while the major testing contracts for situations such as student body testing at higher ed institutions are often being captured by the larger labs.
Payors Respond to Government Guidance Yet Underpayment Continues
In the past three months, payor reimbursement rates have improved, with 17% of claims for COVID-19 antibody and PCR testing being paid below Medicare rates. This is an improvement to the activity XIFIN was seeing in the early phases of the pandemic when payors were improperly denying or mis-paying 43% of these claims, and is evidence of the success of XIFIN’s payor relations’ and its customers’ outreach to educate payors on proper rates and reimbursement rules. However, XIFIN is still seeing a number of both private and public payors improperly denying or underpaying claims, causing financial strain to labs.
The effects of the COVID-19 pandemic on the lab industry have illustrated the importance of maintaining a sound and healthy diagnostic industry across the country and how that might influence future reimbursement rates. Through the CARES Act, clinical labs have a one-year reprieve from the reporting requirements outlined under the Protecting Access to Medicare Act (PAMA) and a one-year delay of rate cuts originally planned for next year. Recent lobbying efforts have shed light on the severe impact of PAMA on labs and the Act’s negative impact on reimbursement rates – giving an indication that the government now has some understanding of the negative impact on diagnostic labs of both past and future PAMA cuts.
A full replay of the UBS audio conference is available here.
About XIFIN
XIFIN is a health information technology company that leverages diagnostic information to improve the quality and economics of healthcare across its portfolio of solutions, including revenue cycle management, laboratory information systems, precision medicine informatics and digital pathology consultation services. Its cloud-based platform offers real-time connectivity, workflow automation, data exchange and actionable insights, linking healthcare stakeholders in the delivery and reimbursement of care. To learn more, visit www.XIFIN.com, follow XIFIN on Twitter and LinkedIn, or subscribe to the XIFIN blog.
View source version on businesswire.com: https://www.businesswire.com/news/home/20200922005762/en/
Source: XIFIN