DWK Life Sciences, the world’s largest manufacturer of precision labware, introduces WHEATON® CompletePAK, a customizable collection of commonly used vials, stoppers and seals that are shipped sterile and delivered ready-to-use with USP certifications for traceability
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20191105005757/en/
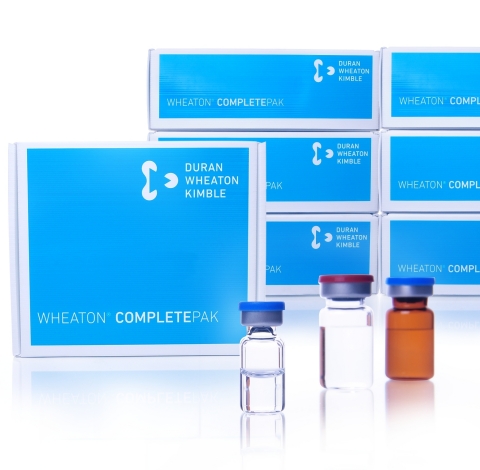
WHEATON® CompletePAK (Photo: Business Wire)
“We saw the advantages of combining vials, stoppers and seals in kits under a single order number for our customer’s workflow, but it really came together when we added our pre-treatment options, USP certifications and Container Closure Integrity (CCI) Testing,” said Rick Schwartz, Senior Vice President of Sales and Marketing at DWK Life Sciences. “Because our CompletePAK kits became so popular, we added ModPAK kits, which contain a larger quantity of ready-to-use, clear glass vial, stopper and seal configurations for larger-scale workflows,” he added.
WHEATON CompletePAK kits (see clear and amber videos) are currently available in over 50 preconfigured, off-the-shelf kits. Kit components include clear or amber glass serum vials in multiple volumes, with a range of stoppers and seals. Pre-treated and sterilized components are packaged in autoclavable bags for entry into cleanroom environments. CompletePAK kits for drug research labs fit under standard fume hoods to maintain aseptic conditions, and kits intended for compounding pharmacies meet or exceed USP Standards allowing compliance with FDA 503c guidelines.
Customers have a choice of starting their CompletePAK order on a convenient web interface WHEATON® CompletePAK or WHEATON® CompletePAK Amber by contacting their DWK Life Sciences representative.
View source version on businesswire.com: https://www.businesswire.com/news/home/20191105005757/en/
Source: DWK Life Sciences