Veeva Systems (NYSE: VEEV) today released its first-ever report examining global trends in medtech clinical trials. According to the 2023 Veeva MedTech Clinical Benchmark Report.
|
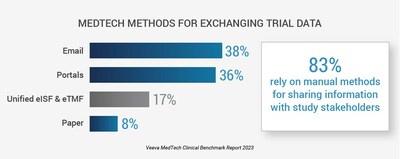
83% use email, portals, and paper to exchange information with study partners, slowing study execution and increasing risk of non-compliance PLEASANTON, Calif., April 19, 2023 /PRNewswire/ -- Veeva Systems, Inc. (NYSE: VEEV) today released its first-ever report examining global trends in medtech clinical trials. According to the 2023 Veeva MedTech Clinical Benchmark Report, on-time data entry and data quality are the top challenges for medtech when working with clinical research sites. This can delay trials and increase compliance risk, making improved collaboration with study sites a critical priority for faster delivery of high-quality data. Research reveals most medtech companies (83%) rely on manual approaches to share information with study stakeholders, such as email, portals, and paper. Manual methods of sharing information slow down study execution and data analysis, increasing the time and costs necessary to complete trial activities. With regulations like EU MDR and IVDR requiring more clinical evidence and performance data, there is a near-term opportunity to advance medtech studies to be faster and more efficient. The report highlights key areas for improvement and progress made in medtech clinical research, including:
"The medtech industry has a significant opportunity to modernize clinical systems and processes for faster access to trial data," said Kevin Liang, vice president, Vault Clinical strategy, Veeva MedTech. "As more organizations prioritize digital clinical technologies, medtech can improve collaboration with stakeholders and drive trial efficiency, productivity, and compliance." The Veeva MedTech Clinical Benchmark study examined how organizations—ranging from emerging to large device and diagnostics companies—manage clinical processes, study site collaboration, and trial data to ensure compliance and speed. This report includes insights from more than 135 clinical medtech professionals worldwide, outlining current challenges and near-term priorities associated with clinical trial conduct. See the full report, which investigates how medtech companies are managing clinical operations, outsourcing, post-market clinical follow-up, and modernization. About Veeva Systems Veeva is the global leader in cloud software for the life sciences industry. Committed to innovation, product excellence, and customer success, Veeva serves more than 1,000 customers, ranging from the world's largest pharmaceutical companies to emerging biotechs. As a Public Benefit Corporation, Veeva is committed to balancing the interests of all stakeholders, including customers, employees, shareholders, and the industries it serves. For more information, visit veeva.com. Contact: Deivis Mercado
SOURCE Veeva Systems |
||
Company Codes: NYSE:VEEV |