TreeFrog Therapeutics , a French biotech leveraging a unique cell manufacturing technology to advance a pipeline of cell therapies with mass-market potential, and Invetech, a global leader in the development and production of automated manufacturing solutions for cell and advanced therapies, today announced a st
|
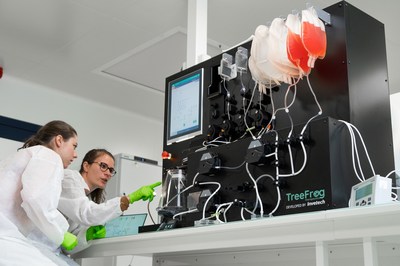
BORDEAUX, France and SAN DIEGO, Oct. 7, 2020 /PRNewswire/ -- TreeFrog Therapeutics, a French biotech leveraging a unique cell manufacturing technology to advance a pipeline of cell therapies with mass-market potential, and Invetech, a global leader in the development and production of automated manufacturing solutions for cell and advanced therapies, today announced a strategic partnership to develop a GMP-compliant device for high-throughput stem cell encapsulation by the end of 2022. Since January 2019, TreeFrog Therapeutics has been collaborating with Invetech to turn its R&D encapsulation set-up into an automated single-use device for industrial bioproduction. "Our C-StemTM technology bridges stem cell biology and biophysics," explained Kevin Alessandri, CEO and CTO of TreeFrog Therapeutics. "We found in Invetech a team eager to confront a new technology, with strong execution capacities, supported by a step-by-step methodology to de-risk the project. As a matter of fact, they managed to deliver the prototype in time in April 2020, right in the middle of the COVID crisis." The beta encapsulation system designed by Invetech meets its technical specifications with a throughput of 1,000 stem cell capsules per second. The first benefit of cell encapsulation is protection against hydrodynamic damages. This is instrumental to amplifying and differentiating fragile cells such as pluripotent stem cells in large-scale bioreactors. The second benefit of the capsule lies in the recapitulation of a biomimetic stem cell niche. In this micro-environment, pluripotent stem cells self-organize in a biomimetic 3D conformation, which promotes fast growth and accurate chromosome segregation. "Today, our C-StemTM technology reduces manufacturing costs by ten-fold, while dramatically improving batch-size, yields and genomic quality. All our efforts are now focused on bringing this technology to the clinic as fast as possible, by advancing a pipeline of cell therapies in co-development with leading pharmaceutical companies. In this context, our partnership with Invetech is critical to secure our roadmap to GMP compliance and to enhance even further the functionality, yield and processing conditions of our encapsulation device," said Maxime Feyeux, co-founder, CEO & CSO of TreeFrog Therapeutics. "TreeFrog approached us with a very novel, early-stage technology that has progressed extremely fast and shows incredible promise," remarked David Kneen, Invetech's Vice President, Cell Therapy. "In under 18 months, our combined teams have transitioned C-StemTM from a bench-top proof-of-concept, to a closed and automated beta production system. It's been a great collaboration driven by our shared vision of commercializing this technology to enable the mass-production of cell therapies for patients in need." Press contacts TreeFrog Therapeutics treefrog.fr Pierre-Emmanuel Gaultier, Marketing & Communication Manager Invetech invetechgroup.com Eeva Routio, Marketing Manager, Brand and Thought Leadership
SOURCE Invetech |