TUCSON, AZ--(Marketwire - April 07, 2011) - SynCardia Systems, Inc., the privately-held manufacturer of the SynCardia temporary Total Artificial Heart, announced today that it set a new Q1 record in 2011, nearly doubling sales from its previous best Q1 in 2010.
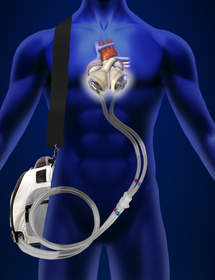
"The company continues to pick up velocity and traction as we work to complete the FDA-approved Investigational Device Exemption clinical study of the Freedom® portable driver," said SynCardia President Michael Garippa. "In Q1, we enrolled an additional 11 patients in the U.S., discharging 6. The Freedom driver, which is also CE approved in Europe, now accounts for more than 8 patient years of life powering the Total Artificial Heart worldwide."
During Q1 2011, SynCardia enrolled 12 new hospitals to its certification program, including Texas Heart Institute, Cedars-Sinai Medical Center and Texas Children's Hospital. Currently, there are 37 SynCardia Certified Centers around the world including 15 in the U.S. There are an additional 32 hospitals currently completing the certification process, 14 U.S. and 18 international.
On April 13, SynCardia is hosting a Clinical Investigator Meeting for the 13.5 lb Freedom driver, the first U.S. portable driver designed to power the Total Artificial Heart both inside and outside the hospital. SynCardia expects triple the attendance from its previous Clinical Investigator Meeting, including surgeons from several international hospitals. The event will be held during the 31st annual meeting of the International Society for Heart & Lung Transplantation (ISHLT) in San Diego.
In its March 2011 issue, Fast Company magazine named SynCardia #20 in its annual list of the World's 50 Most Innovative Companies for "giving mobility to artificial heart recipients." Also in March, the longest running health and wellness series on public television, "Healthy Body, Healthy Mind" produced a 30-minute program about SynCardia's Total Artificial Heart.
About SynCardia Systems, Inc.
SynCardia Systems, Inc. is the Tucson-based manufacturer of the world's only FDA, Health Canada and CE approved Total Artificial Heart: the SynCardia temporary Total Artificial Heart. There have been more than 900 implants of the Total Artificial Heart, accounting for more than 210 patient years of life on the device.
Originally used as a permanent replacement heart, the Total Artificial Heart is currently approved as a bridge to human heart transplant for people dying from end-stage biventricular failure. The Total Artificial Heart is the only device that provides immediate, safe blood flow of up to 9.5 L/min through both ventricles.
For additional information, please visit: http://www.syncardia.com
or follow SynCardia on Twitter - @SynCardia_News
Media Contact:
Don Isaacs
Vice President of Communications
SynCardia Systems, Inc.
Cell: (520) 955-0660