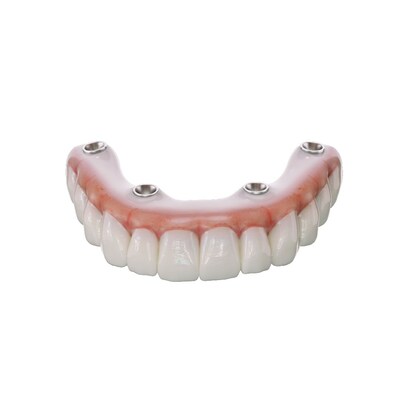
SprintRay Inc. announced the launch of OnX Tough 2, the first and only 3D printing resin with US Food and Drug Administration 510 clearance for fixed, implant-supported denture prosthetics, marking a major step for the dental industry.
- Best-in-class fracture toughness and lifelike aesthetics made possible by proprietary Nanofusion™ technology
- Formulated in new shades Bleach, A1, and A2 for enhanced optical performance
- Joins the comprehensive library of biocompatible 3D printing materials developed by SprintRay for market-leading performance in digital dentistry
LOS ANGELES, Oct. 10, 2023 /PRNewswire/ -- SprintRay Inc., an industry leader in digital dentistry and dental 3D printing solutions, announced today the launch of OnX Tough 2, the first and only 3D printing resin with US Food and Drug Administration (FDA) 510(k) clearance for fixed, implant-supported denture prosthetics, marking a major step for the dental industry.
OnX Tough 2 is part of an integrated, streamlined 3D printing chairside workflow enabling dentists to produce full arch, fixed denture restorations with unparalleled toughness and lifelike translucency for high-quality, same-day smiles. With OnX Tough 2 and the SprintRay ecosystem, dental professionals can 3D print up to 10 fixed dentures in 30 minutes.
The need for implant-supported dental prosthetics is on the rise, with the market expected to grow to $11.54 billion in 2023.1 Unlike conventional removable dentures, fixed hybrid dentures are secured using implants, substantially improving stability, function, and patient satisfaction.2
"Traditional processes for fixed, implant-supported dentures involve up to 6 patient visits and extensive fabrication time," SprintRay CEO and Co-Founder Amir Mansouri, Ph.D., expressed, "We were convinced there had to be a chairside approach. This achievement, marked by the first-ever FDA clearance for a 3D printing material for fixed hybrid dentures, ushers in a new era of same-day restorations while upholding the highest clinical standards, allowing patients to enjoy the benefits of their new smile immediately."
New Chemistry for Dentistry
OnX Tough 2 was formulated using proprietary NanoFusion™ technology, a process that suspends the optimal amount of ceramic in the formulation, which minimizes mixing. This process enhances structural integrity, making restorations printed with OnX Tough 2 exceptionally tough and suitable for demanding dental applications like implant-supported dentures.
Now available in shades Bleach, A1, and A2, OnX Tough 2 builds upon the chemistry from its predecessor resin, OnX Tough, and is formulated with new pigments for enhanced optical performance. These new pigments deliver high-precision shade matching and improved color stability. Fixed dentures printed with OnX Tough 2 have optimal radiopacity for imaging diagnostics, treatment planning, and monitoring healing and progress.
"Implant-supported dentures can be life-changing for patients, improving their speech, chewing, facial balance, and aesthetics, but the fabrication process is time-, resource-, and labor-intensive, deterring many from them," said Keith Klaus, DMD, an innovator in restorative, cosmetic, and implant dentistry with a private practice in Flowood, MS. "FDA-cleared OnX Tough 2 and a streamlined 3D printing chairside workflow marks a watershed moment in the dental industry. It simplifies the process and ushers in a new era of accessibility and convenience for providers and patients alike."
SprintRay will present details of this new technology at the American Academy of Implant Dentistry Conference, November 1-4, 2023. For more information about OnX Tough 2, visit sprintray.com.
About SprintRay
SprintRay designs and manufactures user-friendly and cutting-edge solutions for digital dentistry, including 3D printers, post processing ecosystems, advanced AI-powered software, and innovative materials. Dental care providers can deliver best-in-class service by using SprintRay to speed up delivery and increase customization of care. For more information, visit www.sprintray.com.
Media Contact
press@sprintray.com
1Dental Implants And Prosthetics Global Market Report 2023
2Duong HY et al. Periodontol 2000. 2022 Feb;88(1):201-237. doi: 10.1111/prd.12419.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/sprintray-launches-onx-tough-2-the-first-and-only-3d-printing-resin-fda-cleared-for-fixed-implant-supported-dentures-301951252.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/sprintray-launches-onx-tough-2-the-first-and-only-3d-printing-resin-fda-cleared-for-fixed-implant-supported-dentures-301951252.html
SOURCE SprintRay