HIV remodels its target cells, facilitating spread from the female reproductive tract to the rest of the body, shows a new study at Gladstone Institutes
HIV remodels its target cells, facilitating spread from the female reproductive tract to the rest of the body, shows a new study at Gladstone Institutes
SAN FRANCISCO, May 27, 2020 /PRNewswire/ -- Women represent more than half of new cases of HIV infection worldwide, and most become infected by having sexual intercourse with an infected partner. Yet, women remain understudied in the field of HIV research.
The use of anti-HIV drugs to protect against infection—known as pre-exposure prophylaxis, or PrEP—has shown promise in reducing sexual transmission among men. But its efficacy in preventing transmission to women is less clear.
A team of scientists at Gladstone Institutes and UCSF recently uncovered unique properties of immune cells from women's reproductive tissues that, together with HIV molecular tricks, facilitate the virus's spread in a woman's body. Their findings, published in eLIFE, could inform the design of preventive drugs specially tailored for women.
"Our studies reveal intriguing strategies potentially used by HIV to evade the immune system and spread throughout the woman's body," says Nadia Roan, PhD, a visiting scientist at Gladstone who led the study. "Supplementing PrEP or topical microbicides with drugs that target these processes could improve the overall efficacy of prevention approaches."
Remarkably Receptive Hosts
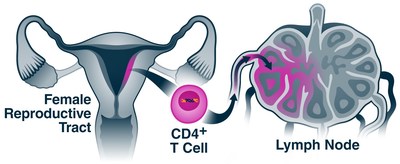
HIV is known to home in on a special type of immune cell called a CD4+ T cell. These cells are found in many tissues, including the lining of the female reproductive tract, and their properties vary depending on their tissue of residence.
"We still lack a fundamental understanding of the features of cells that first become infected upon HIV transmission in women," says Roan, who is also professor of urology at UC San Francisco. "This is partly because cells in the female reproductive tract, unlike those circulating in the blood, are difficult to access."
Roan's team obtained cells of the female reproductive tract from biopsy specimens. They exposed the cells to HIV in the lab to identify the likely targets of the virus in this part of the woman's body. In parallel, the scientists conducted the same infections on blood cells and cells from tonsil biopsies.
While T cells from all three sources became infected by the virus, those from the female reproductive tract were up to 100 times more susceptible to infection than the other cells.
"What's intriguing is that T cells in the female reproductive tract seemed naturally primed to become infected by HIV," says Roan. "While T cells from blood require an extra boost—called activation—to become infected by HIV in the lab, those in the female reproductive tract did not."
Remodeled by the Invader
It is difficult to pinpoint HIV's target cells—in the female reproductive tract and elsewhere—because the virus remodels its host cell after infection. For instance, upon entering a CD4+ T cell, HIV removes the CD4 protein from the cell surface, so the cell no longer looks like a CD4+ T cell.
CD4 is just one of a plethora of proteins a T cell puts on its surface. These proteins serve as the cell's identification card; different cell types have different surface protein profiles, and scientists use these profiles to identify the types of cells present in a tissue or a lab culture.
But because of HIV's remodeling, by the time scientists examine an infected cell, they can't tell whether its surface proteins reflect the cells' original identity or a new identity crafted by the virus.
A few years ago, Roan and her team developed a way to circumvent this problem.
"The virus does not change all the proteins in the cells it infects," says Tongcui Ma, PhD, a postdoctoral researcher in Roan's lab and the first author of the study. "We have a way to monitor a large number of proteins, allowing us to find some that remain unchanged after infection."
These unchanged proteins reveal the identity of the virus's initial targets. By the same token, the scientists also learn what processes the virus remodeled in these cells.
HIV's Agenda—Survive and Spread
Roan's team found that HIV's preferred targets in the female reproductive tract do share some traits with HIV's targets in other tissues. They belong to a class of CD4+ T cells called effector memory cells, which remember previous infections and help fight them off during future encounters.
However, the female reproductive tract cells also display distinctive characteristics compared to blood cells, in particular a high amount of the CCR5 protein on their surface. This protein serves as one of HIV's gateways into cells, and its abundance on the surface of T cells in the female reproductive tract probably explains their high susceptibility to infection.
In addition, the scientists found that a greater variety of T cells appear susceptible to infection in the female reproductive tract samples than in blood samples, which would further help HIV take hold in this tissue.
"We discovered that after infection, T cells in the female reproductive tract display higher amounts of surface proteins known to guide cells toward lymph nodes, which in turn harbor many of the types of cells HIV can infect," says Ma. "We believe this mechanism may help HIV exit the female reproductive tract and enter the lymphatic system, enabling the eventual systemic spread of the virus throughout the woman's body."
Roan's team uncovered other modifications made by HIV that seem aimed at increasing the virus's ability to persist in the face of an active immune system. Infected cells had lower levels of surface proteins that normally help them respond to pathogens. This would cripple their ability to mount a proper defense against invaders, including HIV.
The virus also increased the cells' production of a protein called BIRC5, which protects cells from untimely death. In fact, not only did the virus increase BIRC5, it also seemed to preferentially target cells with higher than average stores of BIRC5.
"Overall, our study suggests that the female reproductive tract is poised for remarkably efficient HIV infection and dissemination," says Roan. "Our findings may help explain the inconsistent effectiveness of oral PrEP or microbicides in protecting women from sexual transmission of HIV."
Scientists can now use these findings to develop strategies that could stem the ability of sexually transmitted HIV to infect women. Roan and her team have already found that a clinically-tested inhibitor of BIRC5 can block HIV's ability to promote the survival of female reproductive tract cells infected in the lab.
"We hope that complementing current interventions with drugs that target features of T cells in the female reproductive tract that make them particularly susceptible to HIV will improve the outcome for women," says Roan.
About the Research Project
Other authors include Xiaoyu Luo and Warner C. Greene from Gladstone Institutes, Gourab Mukherjee from University of Southern California, Nandini Sen from Stanford School of Medicine, Trimble Spitzer from Naval Medical Center, and Ashley F. George and Linda C. Giudice from UC San Francisco.
This work was supported by multiple institutes or centers from the National Institutes of Health (R01 AI127219, R01 AI147777, P01 AI131374, P30 DK063720, S10 1S10OD018040, S10 RR028962, and P30 AI027763).
About the Gladstone Institutes
To ensure our work does the greatest good, Gladstone Institutes focuses on conditions with profound medical, economic, and social impact—unsolved diseases. Gladstone is an independent, nonprofit life science research organization that uses visionary science and technology to overcome disease. It has an academic affiliation with the UC San Francisco.
Media Contact: Megan McDevitt | VP Communications | megan.mcdevitt@gladstone.org | 415.734.2019
1650 Owens Street, San Francisco, CA 94158 | gladstone.org | @GladstoneInst
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/scientists-uncover-ways-hiv-is-sexually-transmitted-in-women-301065816.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/scientists-uncover-ways-hiv-is-sexually-transmitted-in-women-301065816.html
SOURCE Gladstone Institutes