SAB Biotherapeutics announced positive top-line results from the Phase 3 National Institutes of Health’s ACTIV-2 clinical trial that assessed SAB-185 in non-hospitalized people with COVID-19 who were at high risk for severe outcomes.
SAB-185, a human IgG1 (polyclonal) antibody therapeutic candidate, demonstrated benefit in sustained symptom resolution in patients with Omicron variants of SARS-CoV-2
SIOUX FALLS, S.D., April 26, 2023 (GLOBE NEWSWIRE) -- SAB Biotherapeutics (Nasdaq: SABS), (SAB), a clinical-stage biopharmaceutical company with a novel immunotherapy platform that produces specifically targeted, high-potency, fully-human, multi-epitope binding immunoglobulin IgG1 (polyclonal) antibodies without the need for human donors, today announced positive top-line results from the Phase 3 National Institutes of Health’s (NIH) ACTIV-2 clinical trial that assessed SAB-185 in non-hospitalized people with COVID-19 who were at high risk for severe outcomes.
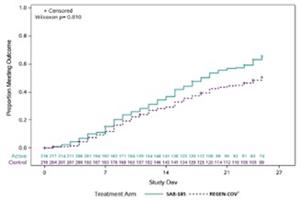
Trial data show that SAB-185 demonstrated benefit in sustained symptom resolution in study participants with COVID-19 caused by Omicron as compared to participants who received a monoclonal antibody combination, REGEN-COV®(casirivimab and imdevimab). Specifically, 66% of participants treated with SAB-185 reached full symptom resolution for at least 4 consecutive days by Day 28, while only 50% of participants on REGEN-COV® met this endpoint (p=0.010; Figure 1, Table 1), and the median time to symptom resolution for at least 4 consecutive days was 7 days shorter for SAB-185. Also, the median time to symptom resolution for at least 2 consecutive days was 6 days shorter for SAB-185, as compared to those who were treated with REGEN-COV® (p=0.021; Figure 2, Table 1). In the non-Omicron population, the median time to symptom resolution for at least 4 consecutive days was 7 days shorter for SAB-185, and the median time to symptom resolution for at least 2 consecutive days was 4 days shorter for SAB-185 than REGEN-COV®, though neither of these analyses met statistical significance.
| Figure 1: Symptom Resolution Over 4 Consecutive Days | Figure 2: Symptom Resolution Over 2 Consecutive Days |
Source: NIH
Table 1: Symptom Resolution for at least 2 days and 4 days
Source: NIH
The primary endpoint of the study was the composite of all-cause hospitalizations and deaths. Following the emergence of the Omicron variant in late 2021, the number of these clinical events dropped significantly. An interim review conducted by the study’s Data and Safety Monitoring Board determined that there would not be enough clinical events in this study, with the number of participants specified in the protocol, to arrive at a conclusive result, and the trial was halted. These data were analyzed and showed the primary endpoint was considered inconclusive for both non-inferiority or superiority in non-Omicron and Omicron patients due to the small number of hospitalizations and deaths. Additionally, grade 3 or higher treatment emergent adverse event rates were similar between SAB-185 and REGEN-COV® arms.
“The ACTIV-2 data is further validation of the potential of SAB’s platform,” said Eddie J. Sullivan, Ph.D., co-founder, President, and Chief Executive Officer of SAB Biotherapeutics. “It shows that the DiversitAb™ platform can open the door to treatments that are potentially more effective and potent and which remain efficacious over longer periods of time versus monoclonal antibodies. This is not surprising, as SAB-185 has been shown to have pseudovirus neutralizing activity1,2 and protection in animal models ranging from Alpha to Omicron variants3.”
Alexandra Kropotova, MD, Chief Medical Officer of SAB Biotherapeutics, said: “In context, these Phase 3 data demonstrate the potential both therapeutic benefits of our fully human multi-epitope binding immunoglobulin antibody therapies as well as their potential to be the modality of choice for COVID-19 and future public health emergencies caused by rapidly mutating viral and bacterial pathogens. This aligns with the FDA’s positive assessment of SAB’s anti-influenza A and B countermeasure. SAB-176 was granted a Fast Track and Breakthrough Therapy designation to treat high risk patients – similar to those at risk for SARS-CoV-2.”
1.https://doi.org/10.1101/2021.08.09.454215
2.https://doi.org/10.1101/2021.02.06.430072
3. https://doi.org/10.1101/2021.07.26.453840
Earlier this month, SAB announced that the FDA had granted both Fast Track designation and Breakthrough Therapy designation to SAB-176, an investigational therapeutic for Type A and Type B influenza illness in high-risk patients, including those who have anti-viral resistant strains. SAB-176 is the first fully-human broadly neutralizing immunoglobulin antibody therapeutic intended to prevent or reduce severe outcomes of Type A and Type B influenza infection in patients at high risk for severe complications, including in patients who are immunocompromised. The Breakthrough Therapy designation was granted to SAB-176 for post-exposure prophylaxis for Type A and Type B influenza illness in high-risk patients, including those who have anti-viral resistant strains.
SAB’s DiversitAb™ platform is a first-of-its-kind technology capable of producing large amounts of fully-human, high-titer, high-avidity multi-epitope binding antibodies across multiple targets without the need for human donors. SAB is leveraging its proprietary platform to discover and develop product candidates with the potential to be first-in-class or best-in-class against complex targets to treat or prevent diseases with significant unmet medical needs. These include infectious respiratory and gastroenterological diseases, immune and autoimmune disorders, and oncology.
Direct support for the development of SAB-185 was provided by the US Department of Defense’s (DoD) Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense (JPEO-CBRND) on behalf of the Office of the Assistant Secretary of Defense for Health Affairs (OASD-HA), and the Defense Health Agency (DHA) and by the Biomedical Advanced Research and Development Authority (BARDA), part of the Department of Health and Human Services (HHS) Office of the Assistant Secretary for Preparedness and Response, under contract number MCDC 2019-448. The Phase 3 clinical trial was sponsored and funded by the National Institutes of Health (NIH) and further information on the trial can be found here.
More information on the SAB-185 COVID therapeutic candidate can be found at: https://www.sab.bio/sab-185/
More information on the SAB-176 influenza therapeutic candidate can be found at: https://www.sab.bio/sab-176/
About SAB Biotherapeutics, Inc.
SAB Biotherapeutics, Inc. (SAB) is a clinical-stage biopharmaceutical company focused on the development of powerful and proprietary immunotherapeutic polyclonal human antibodies to treat and prevent infectious diseases and immune and autoimmune disorders. Our development programs include infectious diseases resulting from outbreaks and pandemics, as well as immunological, gastroenterological, and respiratory diseases that have significant mortality and health impacts on immune compromised patients. SAB has applied advanced genetic engineering and antibody science to develop Transchromosomic (Tc) Bovine™. Our versatile DiversitAb platform is applicable to a wide range of serious unmet needs in human diseases. It produces natural, specifically targeted, high-potency, fully-human polyclonal immunotherapies without the need for human donors. SAB currently has multiple drug development programs underway and collaborations with the US government and global pharmaceutical companies. For more information on SAB, visit: https://www.SAb.bio/ and follow SAB on Twitter and LinkedIn.
Forward-Looking Statements
Certain statements made herein that are not historical facts are forward-looking statements for purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995. Forward-looking statements generally are accompanied by words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “should,” “would,” “plan,” “predict,” “potential,” “seem,” “seek,” “future,” “outlook” and similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements regarding future events, the development and efficacy of our influenza program, C. diff. program, type 1 diabetes program, and other discovery programs, the results, including timing, of the development of SAB-176, SAB-185, SAB-142 and SAB-195, including SAB-176 Fast Track designation and the outcome of and potential future government and other third-party collaborations or funded programs.
These statements are based on the current expectations of SAB and are not predictions of actual performance, and are not intended to serve as, and must not be relied on, by any investor as a guarantee, prediction, definitive statement, or an assurance, of fact or probability. These statements are only current predictions or expectations, and are subject to known and unknown risks, uncertainties and other factors which may be beyond our control. Actual events and circumstances are difficult or impossible to predict, and these risks and uncertainties may cause our or our industry’s results, performance, or achievements to be materially different from those anticipated by these forward-looking statements. A further description of risks and uncertainties can be found in the sections captioned “Risk Factors” in our most recent annual report on Form 10-K, subsequent quarterly reports on Form 10-Q, and other filings with or submissions to, the U.S. Securities and Exchange Commission, which are available at https://www.sec.gov/. Except as otherwise required by law, SAB disclaims any intention or obligation to update or revise any forward-looking statements, which speak only as of the date they were made, whether as a result of new information, future events, or circumstances or otherwise.
CONTACTS:
Investor Relations:
SABIR@westwicke.com
Media Relations:
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/a095499a-435a-4029-863f-28c937c2590a
https://www.globenewswire.com/NewsRoom/AttachmentNg/37d44902-3857-4c92-8aef-1fc6a3afc13f
https://www.globenewswire.com/NewsRoom/AttachmentNg/195a698d-3430-4bd9-82a2-b6ba626265da