Recce Pharmaceuticals Ltd (ASX: RCE), the Company developing new classes of synthetic anti-infectives, is pleased to provide an update on their international SARS-CoV-2 in vitro (organoid) studies undertaken by Path BioAnalytics Inc. (PBA) and The University of Tennessee Health Science Center (UTHSC).
SYDNEY, Australia, Sept. 10, 2020 (GLOBE NEWSWIRE) -- Recce Pharmaceuticals Ltd (ASX: RCE), the Company developing new classes of synthetic anti-infectives, is pleased to provide an update on their international SARS-CoV-2 in vitro (organoid) studies undertaken by Path BioAnalytics Inc. (PBA) and The University of Tennessee Health Science Center (UTHSC).
“We are very pleased with the anti-viral activity against SARS-CoV-2 demonstrated by our two compounds, RECCE® 327 and RECCE® 529 in vitro, and look forward to further success in the forthcoming ferret model studies,” said Non-Executive Chairman Dr. John Prendergast. “As COVID-19 infections and mortalities continue to rise, an effective treatment is critical. Recce’s anti-infective technology is striving to address the global health problem of emerging viral pathogens.”
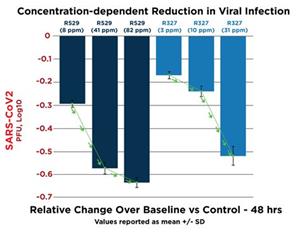
Data indicates concentration-dependent reductions from baseline of the SARS-CoV-2 (COVID-19) virus by R327 and R529 as compared to a control group. The SARS-CoV-2 virus is the cause of the global COVID-19 pandemic. The concentrations utilized were far lower than the suite of pre-clinical data on R327 intravenous infusion program.
A chart accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/ac4d94f6-729a-4bdc-a2f5-2273bca369ff
COVID Organoid Protocol: Organoids comprising human airway epithelial (HAE) cells were infected by inoculation with SARS-CoV-2 virus and incubated at 37 deg C with varying concentrations of R327 (H:31ppm, M:10ppm, L:3ppm) and R529 (H:82ppm, M:41ppm, L:8ppm) and viral load – measured by the number of PFUs (plaque-forming units of virus) – assessed at time points. The Control waspolyethylene glycol (PEG) 200
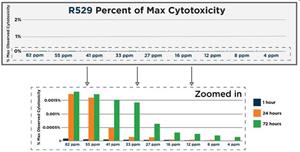
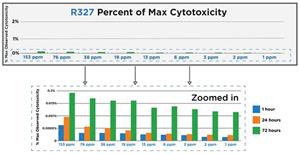
In a separate study, R327 and R529 indicated an excellent toxicity profile with less than 0.25% effect on Vero (monkey) cells at the concentrations tested.
Figures 2 and 3 accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/03cec554-43b4-4984-bcb6-0427da6cdbad
https://www.globenewswire.com/NewsRoom/AttachmentNg/3d618233-5a0a-4fc0-82eb-6c40949ad0b9
Cytotoxicity Testing in Vero Cells Protocol: The cytotoxicity of R327 (153 ppm, 76 ppm, 38 ppm, 19 ppm, 13 ppm, 6 ppm, 3 ppm, 2 ppm, 1 ppm) and R529 (82 ppm, 55 ppm, 41 ppm, 33 ppm, 27 ppm, 16 ppm, 12 ppm, 8 ppm, 4 ppm) across a range of concentrations was assessed in a Vero cell luminescence assay, and cell viability measured at time-points of 1 hour, 24 hours and 72 hours incubation, using a Control of untreated healthy Vero cells. At all time points and concentrations measured, both RECCE® compounds demonstrated minimal cytotoxic effects, with more than 99% of tested cells retaining their viability.
Based on these results, U.S. researchers have recommended that the Company should advance research of both R327 and R529 and has secured testing of the compounds in a gold-standard in-vivo COVID-19 infection study in animals (ferrets). The method of administration in the U.S. study will be intranasal administration to target viral infection in the airways/lungs.
Should a separate study of R327 against COVID-19 in Australia be successful, it would be expected to assess administration by intravascular administration, providing optimum administration route and dose/concentration for potential human COVID-19 therapy. The U.S. ferret study is expected to begin this month and be completed prior to the end of 2020.
Whilst Recce is delighted by the results, further testing must be completed before either (or both) compounds may be deemed safe or effective as a treatment of SARS-CoV-2.
The Company would like to acknowledge University of Tennessee Health Science Center for performing the experiments.
About Recce Pharmaceuticals Ltd
Recce Pharmaceuticals Ltd (ASX: RCE) is pioneering the development and commercialisation of New Classes of Synthetic Anti-Infectives designed to address the urgent global health problems of antibiotic resistant superbugs and emerging viral pathogens.
Recce’s anti-infective pipeline is unique and comprised of broad-spectrum synthetic polymer antibiotics RECCE® 327 and RECCE® 435, and RECCE® 529 for viral infections with unique mechanisms of action against hyper-mutation on bacteria and viruses, respectively.
Patented lead candidate RECCE® 327 has been developed for the treatment of blood infections and sepsis derived from E. coli and S. aureus bacteria – including their superbug forms. Recce’s new antibiotic compound, RECCE® 435, has been formulated for oral use.
The FDA has awarded RECCE® 327 Qualified Infectious Disease Product designation under the Generating Antibiotic Initiatives Now (GAIN) Act – labelling it for Fast Track Designation, plus 10 years of market exclusivity post approval.
Recce wholly owns its automated manufacturing, ready to support first-in-human clinical trials. Recce’s anti-infective pipeline seeks to exploit the unique capabilities of RECCE® technologies targeting synergistic, unmet medical needs.
Corporate Contact
James Graham
Recce Pharmaceuticals Ltd
+61 (02) 8075 4585
James.graham@recce.com.au
Media and Investor Relations (AU)
Andrew Geddes
CityPR
+61 (02) 9267 4511
ageddes@citypublicrelations.com.au
Media and Investor Relations (USA)
Meredith Sosulski, Ph.D.
LifeSci Communications
+1 929 469 3851
msosulski@lifescicomms.com