Late-Breaking Science Presentation | Poster at #ISC26
The information contained herein represents a summary of the abstract presented at the International Stroke Conference (Poster ISC26).
DANBURY, Conn., Feb. 4, 2026 /PRNewswire/ -- Perosphere Technologies Inc. (Perosphere), a medical technology company developing next-generation coagulation diagnostics, today announced a Late-Breaking Science Poster Presentation at the International Stroke Conference (#ISC26), held February 4–6, 2026, at the Ernest N. Morial Convention Center in New Orleans, Louisiana.
Dr. med. Kamran Hajiyev, an interventional neuroradiologist at Stuttgart Hospital's Neuroradiology Clinic in Germany, presented groundbreaking data which may for the first time, provide evidence of a potential prothrombotic state in patients with acute ischemic stroke (AIS) not seen in stroke-free controls. They also suggest that there may be a variable rate of anticoagulant response among patients treated with direct oral anticoagulants (DOACs) - a potential "poor or non-responder" population.
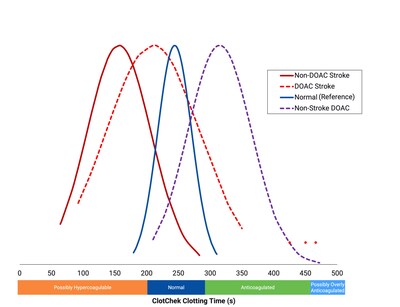
Dr. Hajiyev's team used ClotChek, Perosphere's newly available point-of-care whole blood coagulometer to measure clotting times in this study. ClotChek Clotting Time (CKCT) was assessed in the following patients: AIS +/- DOAC therapy prior to the event, without AIS but on prophylactic DOAC therapy, and normal controls (no AIS, no DOAC). Results showed that both anticoagulated and non-anticoagulated AIS patients showed significantly shorter clotting times (i.e., more coagulation activity) than non-AIS controls (Figure 1).
Among patients receiving DOACs, the anticoagulant response differed markedly between those with AIS and those without AIS. Despite ongoing DOAC therapy, patients with AIS exhibited only minimal anticoagulant activity compared with non-AIS patients on DOACs, suggesting a potentially inadequate anticoagulation effect during stroke.
"This research provides critical insights on the potential to transform stroke care. For the first time, it offers evidence of a prothrombotic state in stroke patients that may not be detected by standard testing, with significant implications for protecting these very vulnerable patients," said Professor Hans Henkes, Medical Director of the Neuroradiology Clinic at Klinikum Stuttgart. "With the ClotChek information available in the angio suite we start to understand much better the (sometimes missing) efficacy of DOACs and the patient-specific coagulation features."
In stroke-free patients on DOACs, CKCT values closely tracked trough and peak drug levels on a population means basis as measured by calibrated anti-factor Xa (anti-Xa), for rivaroxaban (R² = 0.99) and apixaban (R² = 0.98). By contrast, AIS patients on DOACs showed a dissociation between the assays: despite therapeutic anti-Xa levels, CKCT values were below normal, demonstrating lack of meaningful correlation (R² < 0.1). These results suggest that standard anti-Xa assays may overstate the true anticoagulant effect in DOAC patients with breakthrough strokes. Together, the findings highlight a potential poor or non-responder DOAC population with preserved prothrombotic potential and underscores important clinical implications for acute AIS management.
"These highly novel findings have significant implications for design of new studies to improve care and outcomes in patients with acute ischemic stroke, particularly in understanding why some patients suffer strokes despite anticoagulation therapy," said Dileep R. Yavagal, MD, Director of Interventional Neurology and Co-Director of Neuroendovascular Surgery at the University of Miami and Jackson Memorial Hospitals and Clinical Professor of Neurology and Neurosurgery.
"We commend the investigators for demonstrating ClotChek's ability to better inform clinical decision-making in AIS patients," said Sasha Bakhru, PhD, co-author and CEO of Perosphere. "These findings reinforce the value of our point-of-care ClotChek coagulometer in delivering a rapid assessment of anticoagulant effect, particularly for patients on DOAC therapy with the potential to improve care and outcomes."
Oral Poster Presentation
Title: Variable Rate of Anticoagulation Response to Direct Oral Anticoagulants (DOACs) in Acute Ischemic Stroke: The Potential of Point-of-Care DOAC Anticoagulation Measurement
Presenter: Dr. med. Kamran Hajiyev
Date: Wednesday, February 4, 2026
Time/Location: 12:30–2:30 p.m. CST
For more information, visit #ISC26: https://professional.heart.org/en/meetings/international-stroke-conference.
About Perosphere
Perosphere develops point-of-care diagnostics to support informed clinical decision-making for patients receiving anticoagulant therapy or at risk of bleeding. The Perosphere ClotChek™, is a DOAC sensitive coagulometer designed to rapidly measure whole blood clotting time in 3-8 minutes. Perosphere ClotChek is CE marked in the European Union and is not cleared for use by the FDA in the United States.
Media Contact:
John Friedl
j.friedl@perospheretech.com
+1 (949) 485-0465
Company Contact:
Perosphere
info@perospheretech.com
+1 (475) 218-4600
www.perospheretech.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/variable-rate-of-anticoagulation-response-to-direct-oral-anticoagulants-doacs-in-acute-ischemic-stroke-the-potential-of-point-of-care-doac-anticoagulation-measurement-302676462.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/variable-rate-of-anticoagulation-response-to-direct-oral-anticoagulants-doacs-in-acute-ischemic-stroke-the-potential-of-point-of-care-doac-anticoagulation-measurement-302676462.html
SOURCE Perosphere Technologies Inc.