According to Nova One Advisor, the U.S. generic drugs market size is expected to be worth around 196.90 billion by 2034, increasing from USD 138.24 billion in 2024, representing a healthy CAGR of 3.6% from 2025 to 2034.
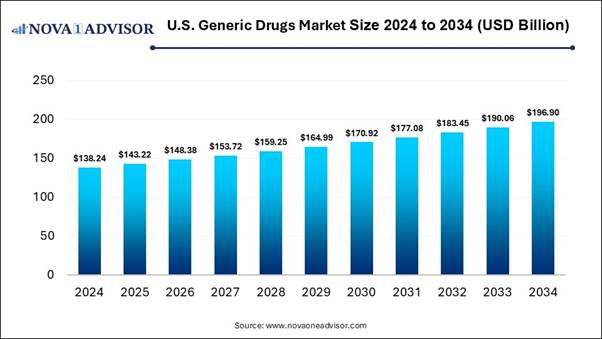
The U.S. generic drugs market is expanding as these types of drugs provide many advantages, not only reduced expenses but also make it simple for people with chronic conditions such as diabetes or high blood pressure and cardiovascular issues to stick to their treatment plans. Generic drugs save a lot of money. They often cost 80% to 85% less than branded drugs.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report@ https://www.novaoneadvisor.com/report/sample/6745
U.S. Generic Drugs Market Highlights:
🔹North America dominated the U.S. generic drugs market with a revenue share in 2024.
🔹Asia Pacific is expected to grow at the fastest CAGR in the market during the forecast period.
🔹By brand, the branded generic drugs segment led the market with the largest revenue share in 2024.
🔹By brand, the pure generic drugs segment is expected to grow at the fastest CAGR in the market during the forecast period.
🔹By route of administration, the oral segment held the largest market share in 2024.
🔹By route of administration, the injection segment is expected to grow at the fastest CAGR in the market during the forecast period.
🔹By distribution channel, the retail pharmacy segment held the highest market share in 2024.
🔹By distribution channel, the online pharmacy segment is expected to grow at the fastest CAGR in the market during the forecast period.
Market Overview and Industry Potential
A generic drug is to pharmaceutical drug that comprises the same chemical substance as a proprietary drug that was formerly protected by chemical patents. Safety, route of administration, strength, performance characteristics, quality, and intended use are the same as branded drugs. These drugs are approved only after a thorough review by the FDA and after a set period of time that the brand product has been on the market.
According to the U.S. Food and Drug Administration, 9 out of 10 prescriptions filled in the United States are for generic medications. Generic medications make it simple for patients to get the medications they require, particularly for chronic health issues. Because they are cost-effective, more people can afford to stay on their treatment plans.
⬥︎ For Instance, In October 2025, the U.S. Food and Drug Administration announced it had launched a novel pilot program to speed up the review process for generic drugs that are tested and manufactured entirely in the United States. The program is designed to encourage companies to invest in domestic drug production and research by offering faster approvals for products made with U.S.-sourced ingredients and tested within the country.
What are Latest Technologies in U.S. Generic Drugs Market?
Technological advancements in the manufacturing of generic drugs, specifically in Artificial Intelligence (AI) and Machine Learning (ML), together with sophisticated manufacturing technologies, are significantly redesigning the generic drug development lifecycle. These novelties are not just incremental development but also show a fundamental transformation, leading to faster timelines, significant expense reductions, improved quality of product, and the ability to allow more complex and targeted formulations, which creates growth opportunities in the generic drugs market.
Latest Trends of the Market
🔹In September 2025, Pfizer Inc. announced a historic agreement with the Trump Administration that will ensure U.S. patients pay lower prices for their prescription medicines while strengthening America’s role as the global leader in biopharmaceutical innovation.
🔹In June 2025, Sanofi and Blueprint Medicines Corporation (Blueprint), a US-based, publicly traded biopharmaceutical company specializing in systemic mastocytosis (SM), a rare immunological disease, and other KIT-driven diseases, entered into an agreement under which Sanofi will acquire Blueprint.
Buy Now Full Report: https://www.novaoneadvisor.com/report/checkout/6745
Report Scope of U.S. Generic Drugs Market
|
Report Coverage |
Details |
|
Market Size in 2025 |
USD 143.22 Billion |
|
Market Size by 2034 |
USD 196.90 Billion |
|
Growth Rate From 2025 to 2034 |
CAGR of 3.6% |
|
Base Year |
2024 |
|
Forecast Period |
2025-2034 |
|
Segments Covered |
By Drug Type, By Brand, By Route of Administration, By Therapeutic Application, By Distribution Channels |
|
Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
|
Key Companies Profiled |
Pfizer Inc, Teva pharmaceuticals USA, Inc, Aurobindo pharma USA, Inc, Sun pharma Inc, Abbott Laboratories Inc, Lupin pharmaceuticals, Inc, Mylan, Dr. Reddy’s, Novartis, Eli Lilly company and Others. |
Recent Advancements in Super Generic Drugs: Market’s Largest Potential
Recently developed super-generic drugs provide many advantages over traditional generic drugs. It plays a significant role in increasing access to cost-effective, enhanced pharmaceuticals while driving invention in drug development and manufacturing. Sympathetic and navigating these government pathways are significant for manufacturers to attain successful entry and address unmet medical needs. Incorporating advanced drug delivery systems, advanced formulations, and therapeutic alterations, they improve efficacy, safety, and patient acquiescence. They offer extra advantages above regular generics, like better safety, efficacy, and suitability of use. This technology encapsulates drugs within minute particles to allow targeted and controlled release, improving effectiveness and lowering adverse effects.
⬥︎ For Instance, In July 2025, RaySearch Laboratories AB (publ) announced that AKSM/Oncology has selected RayCare oncology information system and RayStation treatment planning system for Advanced Radiation Therapeutics (ART), a new partnership with Urology Associates of The Central Coast in San Luis Obispo in California, USA. The center is expected to open in March 2026 and will offer patients inclusive treatment using different techniques. The novel center will use RayStation and RayCare together with its Varian TrueBeam linear accelerators.
By Regional Insight
The increasing demand for generic drugs in the U.S. is expanding due to rising healthcare expenses, expiring patents on blockbuster drugs, and supportive regulatory guidelines. These factors all make generic drugs a cost-effective option for customers and a significant tool for managing inclusive healthcare expenditure. As total medical care expenses continue to rise in the U.S., there is considerable pressure on insurers, consumers, and the government to find more cost-effective solutions, which drives the growth of the market.
⬥︎ For Instance, In October 2025, the U.S. Food and Drug Administration (FDA) announced a new pilot prioritisation program for the review of abbreviated new drug applications (ANDAs). The program is designed to support investment in U.S.-based drug manufacturing and research and development, and to strengthen the domestic pharmaceutical supply chain by offering faster reviews to generic drug companies that conduct testing and manufacturing in the United States.
U.S. Generic Drugs Market Segmentation Analysis:
By Brand
The branded generic drugs segment dominates in the U.S. generic drugs market, as branded generics occupy a different position in medicinal assessing structures, it's priced below original branded drugs but more than unbranded generics. This mid-way assessing approaches enables cost savings as compared to originator brands while generating higher margins than unbranded substitutes. For medical care systems, branded generics provide significant cost suppression opportunities. Branded generics pay expressively to medication accessibility and affordability.
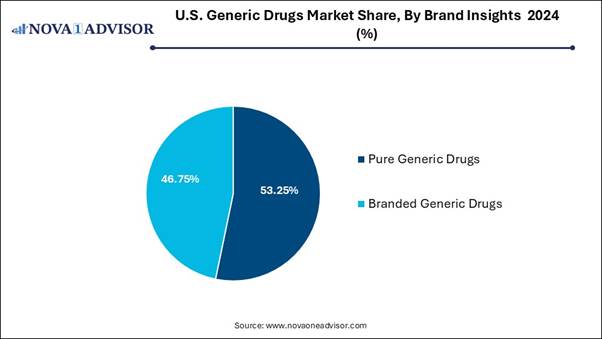
On the other hand, the pure generic drugs segment is expected to grow significantly during the forecast period, as these drugs provided by a presumed generic medicine supplier are an effective and FDA-approved choice for patients. Such drugs are matched to their brand-name equivalent in many categories, including strength, dosage, safety, form, quality, intended use, and performance characteristics. Generic drugs are an easy and inexpensive substitute for treating numerous people who have to take prescription drugs.
By Route of Administration Analysis
The oral segment dominated the market in 2024, as it is a suitable, affordable, and most commonly used medication administration route. Its advantages include good patient compliance, safety, pain avoidance, ease of ingestion, and versatility to accommodate numerous types of drugs. It is the most preferred route, due to its benefits, like non-invasiveness, patient compliance, and suitability of drug administration. Because of its non-invasive nature, many people are more expected to take their medication as prescribed, enhancing treatment results.
On the other hand, the injection segment is expected to grow at the fastest CAGR in the market during the forecast period, as injections are absorbed more quickly and more consistently, given to people who can't take drugs orally, and are also the only route obtainable for administering some medications. The drug is delivered directly in the systemic circulation through intravenous injection, confirming 100% bioavailability and instant attainment of maximum plasma concentration. Intravenous route of administration needs an adequately functioning cannula for admittance, labor-intensive associated with other routes, is exacting due to dosing challenges.
By Therapeutic Application Analysis
The diabetes segment dominated the market in 2024, as generic medications in diabetes make it simple for patients to get the medications they require, particularly for long-term health challenges. Generic medicines are characteristically less expensive than their brand-name counterparts due to lower development expenses. Generic drugs are a therapeutically suitable substitute to their brand-name counterparts and are proven to be medically effective and safe.
On the other hand, the cardiovascular diseases segment is expected to grow at the fastest CAGR in the market during the forecast period, as in cardiovascular diseases, substituting generic drugs in the place of brand-name medicine is a mechanism that enhances the value of pharmaceutical expenditure. Much research and meta-analyses confirmed the medical equivalence of generic and brand-name cardiovascular drugs concerning both safety and efficacy.
By Distribution Channel Analysis
The retail pharmacy segment dominated the market in 2024, as these types of pharmacies play a significant role in medical care by offering convenient access to medicines, a broad range of products, and appreciated services. These pharmacies serve as trusted hubs where patients obtain prescription medications, over-the-counter products, various health-related services, and health advice. Their significance in encouraging public health and wellness cannot be overstated, as they bridge the gap between healthcare providers and patients, offering convenience, expertise, and personalized care.
On the other hand, the online pharmacy segment is expected to grow at the fastest CAGR in the market during the forecast period, as online pharmacy is a simple and convenient mode to purchase medicines. It is specifically advantageous for patients who live far away from drug stores, old, physically challenged, and disabled patients who cannot step out to fetch drugs for themselves, working doctors who have a busy schedule, and patients who live in isolated and rural zones. A person can place an order anytime and anywhere and have it brought at the most suitable time. Patients can readily place their needs for medicine by uploading the scanned copy of their prescription.
Immediate Delivery is Available | Get Full Report Access@ https://www.novaoneadvisor.com/report/checkout/6745
U.S. Generic Drugs Market Companies:
• Teva Pharmaceuticals USA, Inc
• Aurobindo Pharma USA, Inc
• Abbott Laboratories Inc
• Lupin Pharmaceuticals, Inc
• Mylan
• Dr. Reddy’s
• Novartis
• Eli Lilly Company
What is Going Around the Globe?
🔹In August 2025, Natco Pharma launched a generic medication to treat high blood pressure in the lungs in the US with 180-day exclusivity. The company has launched Bosentan tablets for oral suspension (32 mg), a generic version of Tracleer tablets by Actelion Pharmaceuticals US Inc.
🔹In August 2025, Mallinckrodt plc and Endo, Inc. announced that they had completed their merger to create a global, scaled, diversified therapeutics leader. The combined company is well-positioned to continue growing its brand portfolio across a wide range of therapeutic areas of significant unmet need, including endocrinology, gastroenterology, hepatology, neonatal respiratory critical care, nephrology, neurology, pulmonology, ophthalmology, orthopedics, rheumatology, and urology.
🔹In March 2025, the U.S. Food and Drug Administration said on Friday it has launched a new pilot program to speed up the review process for generic drugs that are tested and manufactured entirely in the United States. The program is designed to encourage companies to invest in domestic drug production and research by offering faster approvals for products made with U.S.-sourced ingredients and tested within the country.
You can place an order or ask any questions, please feel free to contact at sales@novaoneadvisor.com | +1 804 441 9344
Related Report
🔹Alzheimer’s Disease Diagnostics Market- https://www.biospace.com/press-releases/alzheimers-disease-diagnostics-market-size-to-reach-usd-25-53-billion-by-2034
🔹U.S. DNA Manufacturing Market - https://www.biospace.com/press-releases/u-s-dna-manufacturing-market-size-to-reach-usd-14-75-billion-by-2034
🔹U.S. Medical Device Contract Manufacturing Market - https://www.biospace.com/press-releases/u-s-medical-device-contract-manufacturing-market-size-to-hit-usd-62-42-billion-by-2034
🔹Human DNA Vaccines Market- https://www.biospace.com/press-releases/human-dna-vaccines-market-size-expected-to-hit-usd-691-23-million-by-2034
🔹U.S. Gastric Cancer Diagnostics Market- https://www.biospace.com/press-releases/u-s-gastric-cancer-diagnostics-market-size-share-and-growth-report-2034
🔹U.S. Pharmaceutical CDMO Market- https://www.biospace.com/press-releases/u-s-pharmaceutical-cdmo-market-size-to-hit-usd-83-86-billion-by-2034
🔹Red Biotechnology Market- https://www.biospace.com/press-releases/red-biotechnology-market-size-to-reach-usd-1-511-28-billion-by-2034
Segments Covered in the Report
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc. has segmented the U.S. generic drugs market
By Brand
• Pure Generic Drugs
• Branded Generic Drugs
By Route of Administration
• Oral
• Injection
• Cutaneous
• Others
By Therapeutic Application
• Central Nervous System (CNS)
• Cardiovascular
• Diabetes
• Infectious Diseases
• Musculoskeletal Diseases
• Respiratory
• Oncology
• Others
By Distribution Channels
• Retail Pharmacy
• Hospital Pharmacy
• Online and Others
Buy Now Full Report: https://www.novaoneadvisor.com/report/checkout/6745
About-Us
Nova One Advisor is a global leader in market intelligence and strategic consulting, committed to delivering deep, data-driven insights that power innovation and transformation across industries. With a sharp focus on the evolving landscape of life sciences, we specialize in navigating the complexities of cell and gene therapy, drug development, and the oncology market, enabling our clients to lead in some of the most revolutionary and high-impact areas of healthcare.
Our expertise spans the entire biotech and pharmaceutical value chain, empowering startups, global enterprises, investors, and research institutions that are pioneering the next generation of therapies in regenerative medicine, oncology, and precision medicine.
Web: https://www.novaoneadvisor.com/
Contact Us
USA: +1 804 420 9370
Email: sales@novaoneadvisor.com
For Latest Update Follow Us: LinkedIn

