- The OmniVu is a revolutionary dual-optic lens designed to restore dynamic visual function by harnessing the eye's natural accommodative mechanism while preserving its anatomical integrity.
- Global clinical trials show promising long-term predictability and stability in more than 75 treated eyes.
CAMPBELL, Calif.--(BUSINESS WIRE)--Atia Vision, Inc., a Shifamed portfolio company, today announced it has received Investigational Device Exemption (IDE) approval from the U.S. Food and Drug Administration (FDA) to begin a traditional feasibility clinical study of its OmniVu™ Lens System. This novel intraocular lens is designed to restore dynamic range of vision following cataract surgery, going beyond the capabilities of current presbyopia-correcting lenses.
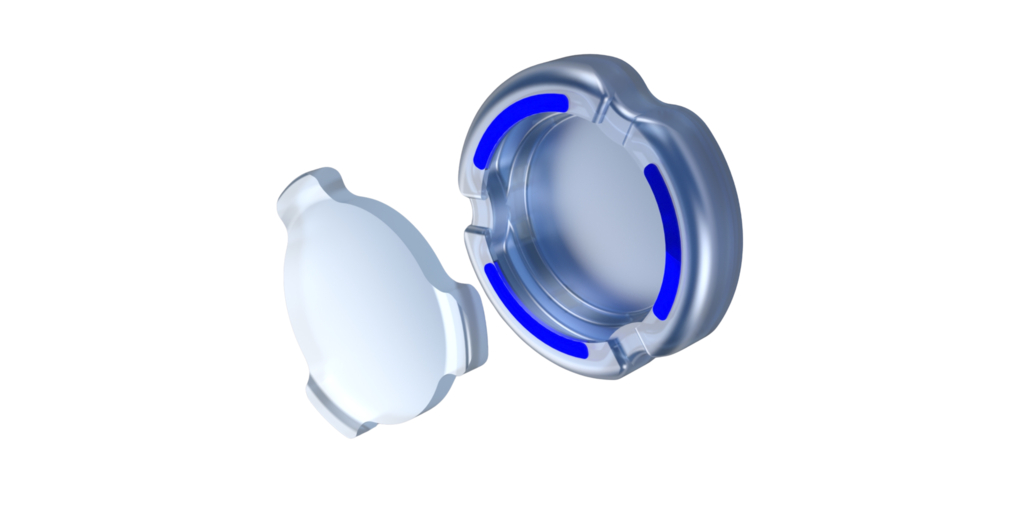


"This IDE approval marks a pivotal milestone in our mission to transform the standard of care for cataract patients," said Mariam Maghribi, CEO of Atia Vision. "OmniVu was developed to solve for the limitations of both accommodative and traditional lenses. Our technology represents a significant advancement in lens design by addressing both optical and functional compromises found in current solutions."
The OmniVu Lens System is comprised of two distinct components: a fluid-filled shape-changing base that provides focusing capability, or zoom function, and a front optic that docks into the base provides the optical power. Additionally, engineered with a more physiologic shape to fill the capsular bag, the OmniVu Lens System is expected to preserve the eye's anatomic integrity and elasticity. This advanced design aims to provide patients with a more natural visual experience across all distances that continues to perform over time.
"The final frontier for lens surgery is an intraocular lens that combines outstanding distance vision with dynamic focusing that maintains excellent image quality," said Dr. George Waring, IV, medical advisory board member for Atia Vision. "The global outcomes to date demonstrate the real potential the OmniVu Lens System has to address the unanswered opportunities with current lens technology, such as a continuous visual range, refractive predictability and stability, all while minimizing visual trade-offs."
The OmniVu Lens System has been evaluated internationally in both first-in-human and feasibility clinical trials with over 75 lenses implanted to date and follow-up extending to three years. Clinical results thus far demonstrate continuous range of focus from far through near with 100% of patients achieving 20/20 or better uncorrected distance vision. Preliminary contrast sensitivity and patient-reported outcomes data demonstrate the potential for parity with monofocal lenses, the current standard for visual quality after cataract surgery.
Approximately 94 million people worldwide are visually impaired due to cataracts.1 Traditional monofocal and presbyopia-correcting lens options are static designs that force patients to compromise between high-quality vision with narrow focus or range of vision with reduced quality. Despite 100% of patients needing to see clearly from near to far, presbyopia-correcting lens adoption remains low at only 6.2% globally.2
About Atia Vision, Inc.
Atia Vision is developing a modular presbyopia-correcting intraocular lens to address the full range of vision for patients with cataracts and presbyopia. The accommodating lens technology is designed to provide superior visual outcomes with an excellent safety profile and be uniquely customizable to address patients' evolving vision needs. Founded in 2014, Atia Vision's technology originated from the Shifamed portfolio, a Silicon Valley-based medical innovation hub. For more information, visit www.atiavision.com.
About Shifamed LLC
Founded by serial entrepreneur Amr Salahieh, Shifamed LLC is a highly specialized medical innovation hub focused on developing advanced solutions that accelerate time to market, reduce risk, increase impact, and forge a path toward a world where patients are able to lead longer, healthier lives. To learn more about Shifamed, please visit www.shifamed.com.
The OmniVu™ Lens System is an investigational device and is not approved for sale in any market.
References:
- Blindness and Vision Impairment Report (who.int)
- 2021 Market Scope IOL Report
Contacts
Media Contact:
Silvana Guerci-Lena
Phone: 508-808-8993
Email: media@launchlabpartners.com




