CAR-T cell therapy has demonstrated remarkable success in treating hematologic malignancies and is now expanding into solid tumors. On June 1, 2025, The Lancet published positive results from the world’s first randomized controlled trial of CLDN18.2 specific CAR-T in gastric cancer, led by Professor Lin Shen’s team at Peking University.
CAR-T cell therapy has demonstrated remarkable success in treating hematologic malignancies and is now expanding into solid tumors. On June 1, 2025, The Lancet published positive results from the world's first randomized controlled trial of CLDN18.2 specific CAR-T in gastric cancer, led by Professor Lin Shen’s team at Peking University. The study reported significantly prolonged progression-free survival and a 44% improvement in overall survival. Recently, CAR-T has also shown great potential in treating autoimmune diseases such as systemic lupus erythematosus and multiple sclerosis.
However, CAR-T development has long faced a persistent challenge: each new CAR targeting a novel tumor antigen requires the iterative development of a fully validated detection reagent to confirm CAR surface expression. This process is not only costly and time-consuming but may also introduce data bias due to insufficient antibody specificity, significantly delaying project timelines.
To address the challenge, Sino Biological has developed anti-whitlow 218 linker rabbit monoclonal antibodies, which specifically binds to the whitlow 218 linker sequence (GSTSGSGKPGSGEGSTKG). The antibodies are independent of antigen specificity, making it applicable across multiple CAR constructs and target antigens.
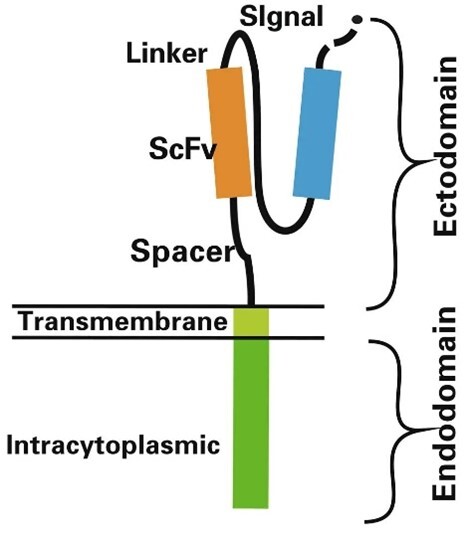
A CAR (Chimeric Antigen Receptor) is a genetically engineered receptor that combines the antigen-binding ability of an antibody with T-cell activation capabilities. The CAR structure comprises four main components:
1) Extracellular Antigen-Binding Domain: The core targeting region, typically a single-chain variable fragment (scFv), designed to recognize specific tumor-associated antigens with high specificity.
2) Hinge Domain: Spans the extracellular antigen-binding domain and the transmembrane domain, providing flexibility and supporting proper antigen-binding conformation.
3) Transmembrane Domain: Anchors the CAR structure into the T-cell membrane, ensuring stability and functionality.
4) Intracellular Signaling Domain: Activates the T cell upon antigen engagement. It usually contains the CD3ζ chain or CD3ε derived from the T-cell receptor, plus costimulatory domains such as CD28 or 4-1BB to enhance persistence and anti-tumor activity.
The CAR positive rate is a critical metric for evaluating CAR-T product potency, directly influencing target recognition and cytotoxic efficacy. Monitoring CAR expression in peripheral blood or tissues post-infusion provides insights into in vivo expansion and persistence, informing treatment response, durability, and potential relapse risk. Dynamic CAR expression monitoring also helps assess immunogenicity and safety. Thus, a sensitive, reliable, and universal CAR detection system is vital for developing and evaluating CAR-T cell therapies.
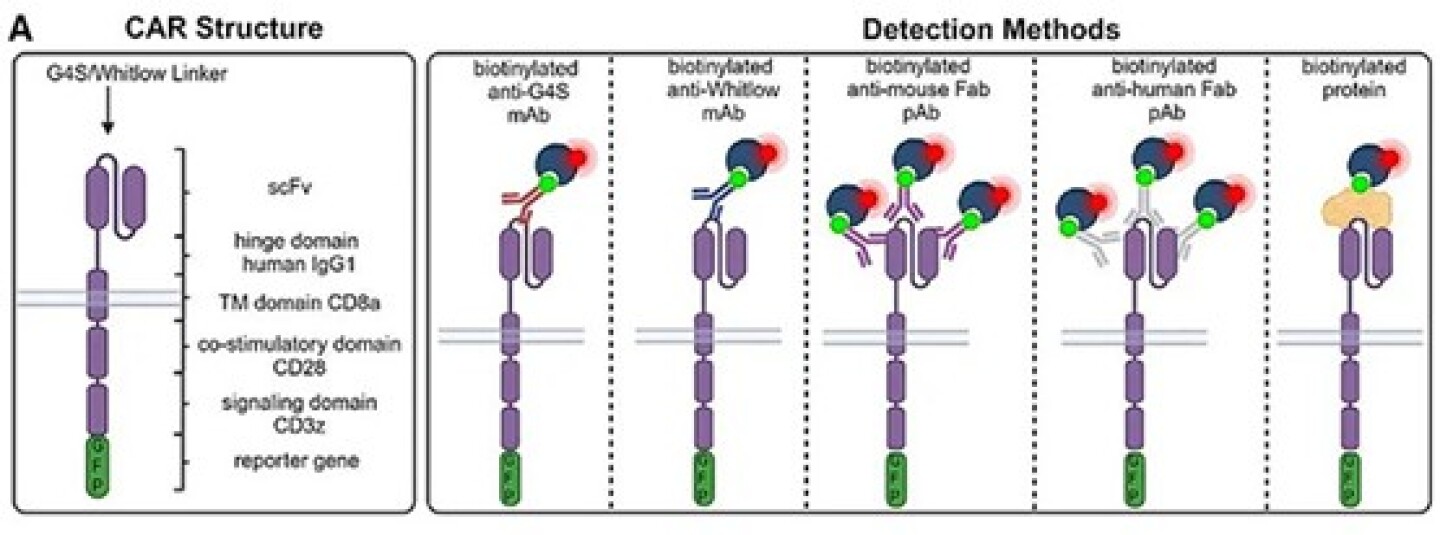
Currently, approved CAR-T products target CD19 or BCMA, making flow cytometry with recombinant BCMA or CD19 protein the standard detection method. Although highly specific, this approach is limited to single targets and is unsuitable for CAR-T screening and preclinical development.
Several CAR detection methods have been developed, including Protein L (which binds immunoglobulins) or Fc-binding polyclonal antibodies. Protein L binds only to kappa light chains and fails to detect CARs containing lambda chains. Polyclonal antibodies may cross-react with patient IgG, causing false positives. Anti-idiotype antibodies offer high specificity and sensitivity by targeting unique idiotopes in the CAR's antigen-binding region, but they are challenging to develop and produce.
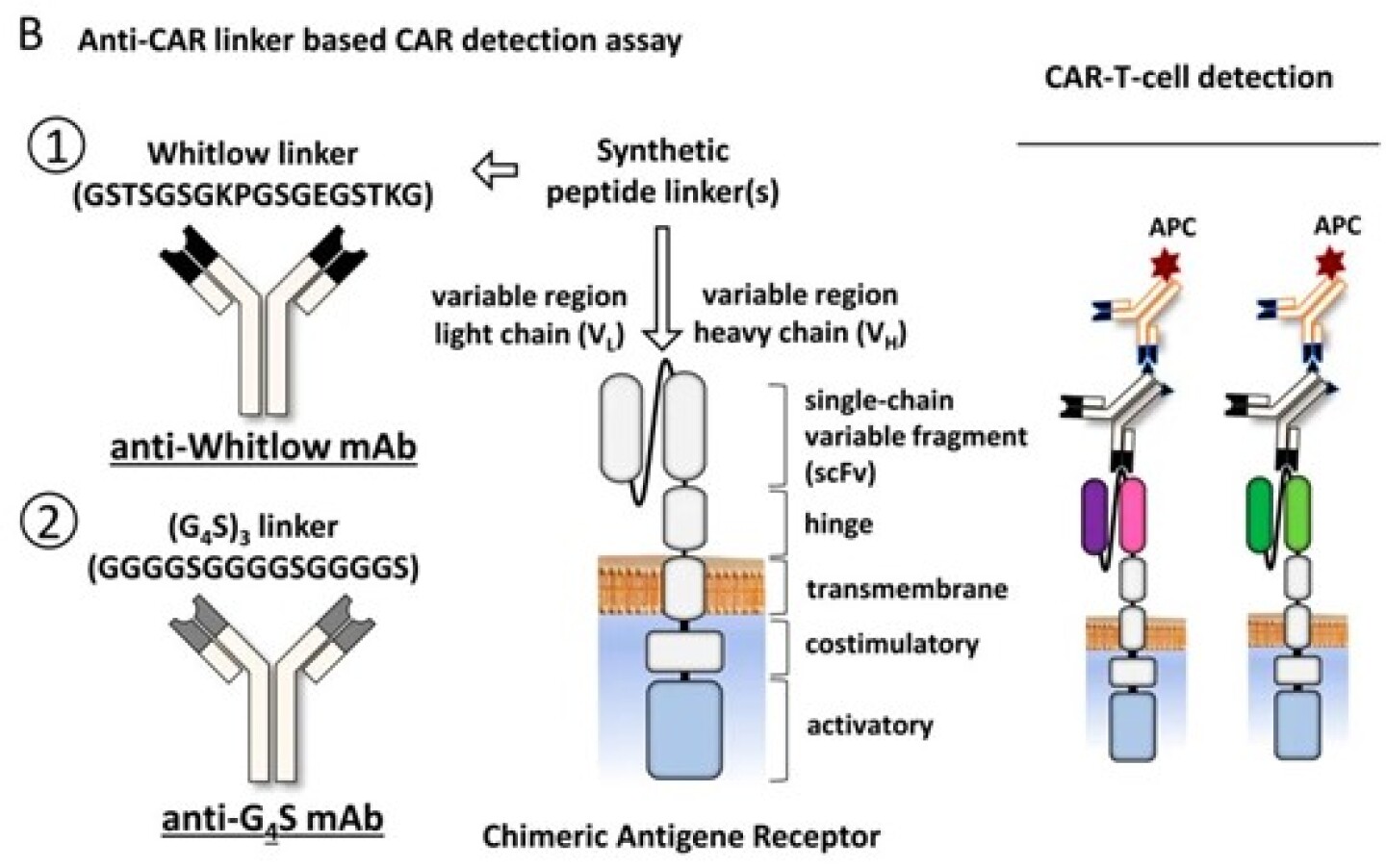
Anti-CAR linker monoclonal antibodies offer a universal strategy for CAR detection. Most CARs incorporate an scFv domain connected via flexible linkers such as Whitlow 218 or G4S. These standardized linker sequences provide a consistent and well-defined epitope. Regardless of the CAR’s target antigen (e.g., CD19, BCMA, Claudin18.2, or CD22), anti-linker antibodies can detect CAR expression accurately, significantly improving universality and consistency compared to antigen-specific detection methods.
The 218 linker, known for its good flexibility and stability, is used in nearly 90% of scFv-based CAR constructs. First described by Whitlow et al. in 1993, its amino acid sequence (GSTSGSGKPGSGEGSTKG) promotes correct folding between VH and VL domains, enhancing scFv stability and function.
Antibodies specifically targeting the linker region enable efficient and standardized CAR detection, offering five key advantages:
Sino Biological provides a diverse range of products and services for CAR detection, supporting CAR-based cell therapy development. For more information, please visit https://www.sinobiological.com/resource/featured-review/car-linker
1. Katharina Schindler, et al. Linker-specific monoclonal antibodies present a simple and reliable detection method for scFv-based CAR NK cells. Molecular Therapy: Methods & Clinical Development. 2024, https://doi.org/10.1016/j.omtm.2024.101328
2. Grahnert, A et al. Evaluation of Anti-CAR Linker mAbs for CAR T Monitoring after BiTEs/bsAbs and CAR T-Cell Pretreatment. Biomedicines 2024, https://doi.org/10.3390/biomedicines12081641
3. Zhiwei Wang, et al. CAR-T therapy dilemma and innovative design strategies for next generation. Cell Death and Disease, 2025. https://doi.org/10.1038/s41419-025-07454-x
However, CAR-T development has long faced a persistent challenge: each new CAR targeting a novel tumor antigen requires the iterative development of a fully validated detection reagent to confirm CAR surface expression. This process is not only costly and time-consuming but may also introduce data bias due to insufficient antibody specificity, significantly delaying project timelines.
To address the challenge, Sino Biological has developed anti-whitlow 218 linker rabbit monoclonal antibodies, which specifically binds to the whitlow 218 linker sequence (GSTSGSGKPGSGEGSTKG). The antibodies are independent of antigen specificity, making it applicable across multiple CAR constructs and target antigens.
What is CAR Structure?
1) Extracellular Antigen-Binding Domain: The core targeting region, typically a single-chain variable fragment (scFv), designed to recognize specific tumor-associated antigens with high specificity.
2) Hinge Domain: Spans the extracellular antigen-binding domain and the transmembrane domain, providing flexibility and supporting proper antigen-binding conformation.
3) Transmembrane Domain: Anchors the CAR structure into the T-cell membrane, ensuring stability and functionality.
4) Intracellular Signaling Domain: Activates the T cell upon antigen engagement. It usually contains the CD3ζ chain or CD3ε derived from the T-cell receptor, plus costimulatory domains such as CD28 or 4-1BB to enhance persistence and anti-tumor activity.
Why Measuring CAR Expression is Important?
Currently, approved CAR-T products target CD19 or BCMA, making flow cytometry with recombinant BCMA or CD19 protein the standard detection method. Although highly specific, this approach is limited to single targets and is unsuitable for CAR-T screening and preclinical development.
Several CAR detection methods have been developed, including Protein L (which binds immunoglobulins) or Fc-binding polyclonal antibodies. Protein L binds only to kappa light chains and fails to detect CARs containing lambda chains. Polyclonal antibodies may cross-react with patient IgG, causing false positives. Anti-idiotype antibodies offer high specificity and sensitivity by targeting unique idiotopes in the CAR's antigen-binding region, but they are challenging to develop and produce.
A Universal Strategy: Anti-CAR Linker mAbs
Anti-218 Linker Antibodies: A Powerful Tool for Efficient CAR Detection
Antibodies specifically targeting the linker region enable efficient and standardized CAR detection, offering five key advantages:
- Broad Applicability
Detects any CAR construct containing the Whitlow 218 Linker, eliminating the need for target-specific reagents. - Multiple Conjugate Options
Available conjugated to FITC or PE for flow cytometry. Additional conjugates (APC, AF488, AF647, Biotin) are in development. - Exceptional Specificity
In-house developed rabbit monoclonal antibodies demonstrate minimal non-specific binding to PBMCs (<0.5%). - In Vitro and In Vivo Compatibility
Suitable for assessing transfection efficiency in vitro and monitoring CAR-T expansion dynamically in vivo. - Accelerated Development
Reduces time and resources needed for custom reagent development and validation.
Sino Biological’s Anti-Whitlow 218 Linker Rabbit Monoclonal Antibodies
| Cat# | Conjugate | Application |
| 111774-R0013-F | FITC | FCM |
| 111774-R0013-P | PE | FCM |
| 111774-R0013-D (Pre-order) | AF 488 | FCM |
| 111774-R0013-G (Pre-order) | AF 647 | FCM |
| 111774-R0013-B (Pre-order) | Biotin | FCM |
| 111774-R0013-A (Pre-order) | APC | FCM |
Sino Biological provides a diverse range of products and services for CAR detection, supporting CAR-based cell therapy development. For more information, please visit https://www.sinobiological.com/resource/featured-review/car-linker
References
2. Grahnert, A et al. Evaluation of Anti-CAR Linker mAbs for CAR T Monitoring after BiTEs/bsAbs and CAR T-Cell Pretreatment. Biomedicines 2024, https://doi.org/10.3390/biomedicines12081641
3. Zhiwei Wang, et al. CAR-T therapy dilemma and innovative design strategies for next generation. Cell Death and Disease, 2025. https://doi.org/10.1038/s41419-025-07454-x