NeuClone Pharmaceuticals Ltd. announced it will initiate the Phase I clinical trial of its Stelara® biosimilar in the second half of 2019.
SYDNEY, May 15, 2019 /PRNewswire/ -- NeuClone Pharmaceuticals Ltd. (NeuClone), a clinical-stage biopharmaceutical company exclusively focused on developing high-quality biosimilar products, today announced it will initiate the Phase I clinical trial of its Stelara® (ustekinumab) biosimilar in the second half of 2019.
Stelara® (ustekinumab) is a human monoclonal antibody approved for treatment of moderate to severe plaque psoriasis in adults and children 12 years or older. It is also approved to treat active psoriatic arthritis and moderate to severe Crohn's disease in adults. Stelara® (ustekinumab) is directed against interleukin-12 and -23, naturally occurring proteins that regulate the immune system and immune-mediated inflammatory disorders. Johnson & Johnson reported global Stelara® sales of $5.2 billion for 2018. Stelara® continued its growth in the first quarter of 2019, up 32% from the first quarter of 2018.
NeuClone's Stelara® (ustekinumab) biosimilar is called 'NeuLara'. The multicentre Phase I trial of NeuLara will be conducted in Australia under the Clinical Trial Notification (CTN) scheme of the Therapeutic Goods Administration (TGA). The trial will be a three-arm, randomised, double-blind, single-dose study comparing the pharmacokinetics and safety of NeuLara, to US- and EU-sourced Stelara® in healthy volunteers.
"NeuLara is the second biosimilar to enter clinical development from our 10-biosimilar product portfolio with manufacturing partner Serum Institute of India", said Dr Noelle Sunstrom, Chief Executive Officer at NeuClone. "Our Herceptin® (trastuzumab) biosimilar (called 'NeuCeptin') has recently completed dosing in a similar three-arm Phase I clinical trial in Australia. We look forward to the clinical progress of NeuLara and advancement of other earlier stage biosimilars including our biosimilars already announced of Prolia®/XGEVA® (denosumab), Synagis® (palivizumab) and Perjeta® (pertuzumab)."
NeuLara was developed at NeuClone's facilities using the proprietary NeuMAX® technology and Right from the Start™ development approach. Extensive analytical testing is conducted throughout all stages of development, including X-ray crystallography confirmation of identical NeuLara and Stelara® structures (see Figure 1).
NeuClone representatives will attend the upcoming 2019 BIO conference in Philadelphia from 3-6th June and look forward to discussing biosimilar development and commercialisation opportunities with potential partners.
About NeuClone
NeuClone is Australia's only biosimilar company focused exclusively on developing a pipeline of biosimilar monoclonal antibodies. Six biosimilar products have been disclosed in NeuClone's pipeline that reference Herceptin®, Stelara®, Synagis®, Prolia®/XGEVA®, Perjeta® and Humira®. NeuClone develops biosimilar products using its proprietary NeuMAX® platform that facilitates low-cost manufacture of biologics, while enabling the highest product quality. NeuClone is led by a highly experienced team with state of-the-art integrated facilities based in Sydney, Australia. For more information, please visit www.neuclone.com.
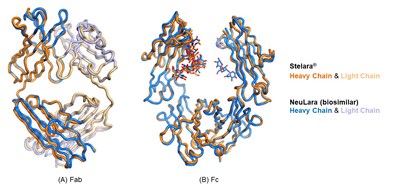
Stelara® and NeuLara X-ray Crystallography
X-ray crystallography is a powerful technique that allows three-dimensional imaging of complex proteins. NeuClone's crystallographic data confirms the identical structures of Stelara® and NeuLara. NeuClone's Right from the Start™ development approach incorporates X-ray crystallography in addition to testing several other critical quality attributes sought by regulators to confirm biosimilarity from the outset and throughout development. This difficult to achieve added dimension, sets NeuClone apart.
Figure 1: X-ray crystallography overlay of Stelara® and NeuLara (A) Fab fragment; and (B) Fc fragment. Comparisons between Stelara and NeuLara indicate structurally identical molecules.
Stelara® is a registered trademark of Johnson & Johnson.
Herceptin® is a registered trademark of Genentech Inc.
Prolia® and XGEVA® are registered trademarks of Amgen Inc.
Synagis® is a registered trademark of MedImmune Inc.
Perjeta® is a registered trademark of Genentech Inc.
Humira® is a registered trademark of AbbVie Inc.
Contact
John Oksinski, Global Head of Business Development - j.oksinski@neuclone.com
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/neuclone-to-initiate-phase-i-clinical-trial-of-stelara-ustekinumab-biosimilar-the-second-biosimilar-from-the-10-product-portfolio-with-serum-institute-300850453.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/neuclone-to-initiate-phase-i-clinical-trial-of-stelara-ustekinumab-biosimilar-the-second-biosimilar-from-the-10-product-portfolio-with-serum-institute-300850453.html
SOURCE NeuClone Pharmaceuticals Ltd.




