IRVINE, Calif., March 4, 2015 (GLOBE NEWSWIRE) -- The German company joimax®, developer of technologies and training methods for minimally invasive endoscopic spinal surgery, today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its Percusys® percutaneous pedicle screw-rod system.
| |||||
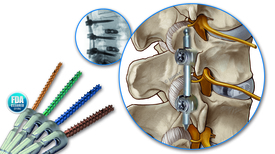
The patented Percusys system is a multi-functional implant for use during spinal stabilization procedures. Its unique screw and instrument design enables a safer and more effective surgical technique with minimal steps for the surgeon. The single, small instrument set allows flexibility to perform surgery through a percutaneous, minimally invasive or open approach.
"During development, the main focus was on the ease-of-use of the system," says Dr. Frank Hassel, specialist for spine surgery from Freiburg, Germany, who was instrumental to the Percusys development. "The idea was to reduce the complexity in instrumentation and simplify stabilization procedures to minimize the potential damage of soft tissues and improve overall patient outcomes."
Percusys implants comprise single packaged, sterile, and pre-assembled pedicle screws with lengthening shaft and set-screw. All screws are color coded according to their diameter, cannulated, fenestrated, self-cutting and self-drilling. Each surgical step is carried out using the lengthening shaft, which is tightly connected to the tulip. The assembly allows for direct manipulation and does not require additional instrumentation. Shearing off the lengthening shaft can be done by a 360-degree rotation of the shaft breaker.
Percusyscan be usedin combination with the joimax® EndoLIF® O-Cage. "This offers an optimal solution for minimally invasive, endoscopic-assisted access to the intervertebral disc," says Wolfgang Ries, CEO and founder of joimax. "Percusys® represents the next logical step in the development of endoscopic surgery and further strengthens the joimax position as an expert in this special market segment."
About joimax
Founded in Karlsruhe, Germany, in 2001, joimaxis one of the leading medical device companies in minimally invasive spinal surgery ("joined minimal access"). The company's U.S. subsidiary was established in Irvine, California, in 2005. The company is primarily focused on the development, production and marketing of technologies and methods for minimally invasive endoscopic spinal surgery. joimaxis active in 40 countries around the globe and its methods have been successfully employed in approximately 130,000 surgeries. With a special focus on education, the company provides surgeons with specialized technique training through the three-step joimax CM3 education program. This program includes visitations, cadaver workshops and live-surgery support.
For more information on the Percusys® system:
Animation video: http://www.joimax.com/en/patients/mediacenter/mediacenter.php
CM3 Program: http://www.joimax.com/en/doctors/education/education_program.php
joimax® homepage: http://www.joimax.com/en/index.php
A photo accompanying this release is available at: http://www.globenewswire.com/newsroom/prs/?pkgid=31167
CONTACT: joimax GmbH Dr. Anja Martin anja.martin@joimax.com Phone +49 (0) 721 25514-212 joimax Inc. Irena Longshore Irena.Longshore@joimaxusa.com Phone +1 949 859 347 2307 Web www.joimax.com ![]()
Help employers find you! Check out all the jobs and post your resume.