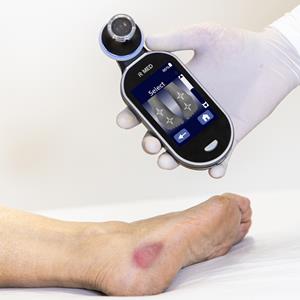
IR-MED Inc. reported highly favorable proof of efficacy data for PressureSafe™, its decision support device which uses infra-red spectroscopy combined with an AI-based algorithm, for the early, non-invasive, and skin color agnostic detection of pressure injuries.
- Incidence of pressure injuries reduced by 50% during the study period
- As a decision support device, PressureSafe, can support early detection of pressure injuries, potentially setting a new standard of care to address a healthcare challenge that costs $26.8 billion annually in the U.S. alone
- Data reported at NPIAP 2024 Annual Conference to key thought leaders and practitioners in the field of pressure injuries
- Nearly 1,500 PressureSafe scans on 154 body locations conducted at hospitals owned by the world’s 2nd largest HMO
Rosh Pina, Israel, Feb. 20, 2024 (GLOBE NEWSWIRE) -- IR-MED Inc., (“IR-MED” or the “Company”) (OTCQB:IRME), developer of a noninvasive artificial intelligence (AI) driven spectrographic analysis technology platform to address significant healthcare needs, today reported highly favorable proof of efficacy data for PressureSafe™, its decision support device which uses infra-red spectroscopy combined with an AI-based algorithm, for the early, non-invasive, and skin color agnostic detection of pressure injuries.
Data from the study conducted at two medical centers owned by Clalit, the world’s second largest health maintenance organization (HMO) and the largest in Israel, Beit Rivka Hospital and Rabin Medical Center, presented at the National Pressure Injury Advisory Panel (NPIAP) 2024 Annual Conference on February 16 and 17, 2024 in San Antonio, Texas. Dr. Gal Maydan of Beit Rivka Hospital Geriatric Rehabilitation Center, and Principal Investigator of the study, presented the data in a poster titled “Near Infra-Red Spectroscopy for early detection of stage 1 pressure injury and deep tissue injury – clinical study results”.
While the current standard of care for the detection of pressure injuries is visual and tactile clinical evaluation, physiological changes below the skin’s surface, including inflammation and interstitial fluids precede changes on the surface. The objective of the study was to evaluate the sensitivity, specificity, and usability of PressureSafe to detect early-stage pressure injuries Stage 1 / suspected deep tissue injuries (sDTI) before skin breakage, compared to standard of care. PressureSafe detected biomarkers and changes in tissue structures under the skin’s surface as they relate to pressure injuries.
The 14-day efficacy portion of the single arm, bi-center study evaluated 38 patients at high risk of pressure injury development. A total of 924 scans were conducted on 154 body locations. Nurses conducting the scans were blinded to PressureSafe’s results, which were encrypted. PressureSafe detected Stage 1 / sDTI pressure injuries with 92% sensitivity and 88% specificity. Additional portions of the study evaluated safety, as well as device calibration and validation. Total data from 66 patients was obtained for safety analysis and no safety signals were identified in 1,493 scans. Based on these data, the study concluded that PressureSafe is a safe, efficient, and valuable method for early detection of pressure injuries.
“These data demonstrate that PressureSafe, an IR-spectroscopy scanner combined with an AI- based algorithm, provides a very good option for detection of early stage pressure injuries - hence facilitating early treatment that is crucial for prevention of complications. This is especially important in diagnosis of people with darker skin colors, where visual and tactile inspection alone may miss early detection,” stated Dr. Maydan. “Our medical staff, including nurses, found the device very easy to use. During the study period the incidence of pressure injuries was reduced by approximately 50% compared to the same period before the study. It is time to integrate advanced technology to augment standard human visual and tactile perception in order to minimize the harmful consequences of pressure injuries. PressureSafe is a device that I can see being used for exactly this purpose.”
Tzur Di-Cori, IR-MED’s CEO, commented, “This robust and impressive data from our collaborative study with Clalit comes at an ideal time as we prepare to enter the U.S. market with PressureSafe. We believe the 92% efficacy findings in real-world data settings at two world-class hospitals will be a big factor in driving adoption in the U.S. We thank Dr. Maydan and his entire team at Clalit for leading this study.”
Yaniv Cohen, PHD, IR-MED’s CSO, added, “In addition to this study in Israel, we look forward to starting a usability study for PressureSafe in the U.S. in collaboration with Methodist Healthcare of San Antonio in the coming months. The Methodist study aims to enroll approximately 50% of patients with darker skin tones in order produce comparative data for PressureSafe’s accuracy as a decision support device in people with lighter and darker skin tones. This is a very important priority for IR-MED, as published studies show black patients in the U.S. suffer disproportionally from pressure injuries, which are harder to detect visually in darker skin tones.”
IR-MED’s clinical and execute team participated at NPIAP 2024 where they demonstrated PressureSafe™ at booth #102.
The poster outlined several advantages of the PressureSafe technology including:
- Non-invasive, multi-biomarker analysis
- Scans for changes at tissue structure under the skin’s surface
- Effective for all skin tones
- AI-based algorithm
- Real-time analysis at the point-of-care
- Personalized medical device
- Cloud based
- Harmless infra-red light
About PressureSafe
PressureSafe, an innovative non-invasive medical device that uses infrared optical spectroscopy and AI, is designed to effectively detect early-stage pressure injuries for all skin tones. Pressure injuries cost the U.S. healthcare system $26.8 billion and lead to 60,000 deaths annually. PressureSafe was found to be over 90% accurate in detecting pressure injuries based on interim results from a multicenter study. The skin-color agnostic device effectively addresses equity in healthcare by using infrared light to detect biomarker changes below the skin’s surface.
About IR-MED
IR-MED Inc. is developing a noninvasive spectrographic analysis technology platform, allowing healthcare professions to detect, measure and monitor, in real time, different molecules in the blood, in human tissue, and in body fluids without invasive procedures. PressureSafe, the first product under development, is a handheld optical monitoring device that is being developed to support early detection of pressure injuries (PI) to the skin and underlying tissue, regardless of skin tone as it calibrates personally to each patient’s skin.
IR-MED’s technology is being developed to allow accurate readings of biomarkers in a non-invasive method, that may provide caregiver the optimal decision support-system in cases where uncertainties disturb physicians in their decision processes.
IR-MED holds patents protecting its technology and innovations in the noninvasive tissue analysis, and in the modeling and analysis of subcutaneous tissue.
PressureSafe is currently undergoing usability studies at multiple medical centers. It is not yet available for commercial use.
Safe Harbor Statement / Forward-Looking Statements
Statements included in this press release, which are not historical in nature, are forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. For example, IR-Med is using forward-looking statements when it discusses the parameters and timing of the study, the expected timing of FDA approval, the potential benefits from its PressureSafe product and the expected timing of the release of its useability study conducted in Israel. Statements relating to the future performance of IR-Med are subject to many factors including, but not limited to, the sufficiency or working capital and our ability to raise the capital needed to fund our development efforts, completion of the development and design of PressureSafe device, results of clinical/useability studies and trials, timing of product development, FDA approval/clearance of products in development, customer acceptance of our products in the market, the introduction of competitive products, the impact of any product liability or other adverse litigation, commercialization and technological difficulties, and the other risks identified in our most recent annual report on Form 10-K filed on March 29, 2023 with the Securities and Exchange Commission. Such statements are based upon the current beliefs and expectations of management and are subject to significant risks and uncertainties. Actual results may differ from those set forth in the forward-looking statements. The forward-looking statements contained in this press release are made as of the date hereof, and we do not undertake any obligation to update any forward-looking statements, whether as a result of future events, new information, or otherwise.
Contact:
Sharon Levkoviz, Chief Financial Officer
Tel: +972 (0) 4 6555054
Attachment