LINCOLNSHIRE, Ill., July 11, 2017 /PRNewswire/ -- Sysmex America, Inc., a leading provider of automated hematology and urinalysis diagnostic testing equipment as well as middleware information systems technology, today announced its latest innovation: a way to make quality assurance easier and more risk free than manual quality control processes. "Sysmex is well known for the innovation that it delivers to the laboratory. BeyondCareSM Quality Monitor elevates quality assurance processes to a new level with automated, continuous monitoring and guidance in an easy-to-use framework," said Ralph Taylor, chief executive officer.
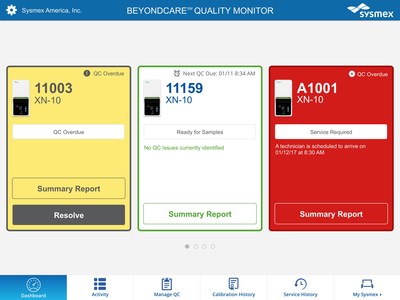
BeyondCare Quality Monitor is a web-based quality control and calibration verification management program that uses evidence-based quality control targets and peer group analytics to improve error detection and minimize false rejection of control results - potentially saving both time and money for the laboratory. The managed QC and calibration program has the potential to identify shifts and trends in QC weeks sooner than traditional methods. Continuous calibration verification occurs every time the QC is analyzed as opposed to every 6 months, further streamlining the error detection process. Reports are integral to the application, so documentation is easier and there is less paper to print and file.
"Sysmex is excited to bring this innovation to the laboratory industry. BeyondCare Quality Monitor eliminates the repetitive and time-intensive nature of quality assurance. One look at the screen and the technologist will see if all is well or if QC has exceeded its target it's all there in green, yellow and red," said Andy Hay, chief operating officer. "This simplifies a critical component of delivering accurate patient results," said Hay. Additional troubleshooting guidelines provided on screen will also assist in resolution.
BeyondCare Quality Monitor will be standard on the new line of Sysmex XN-L automated hematology analyzers available in the United States. There are plans to extend the new service to other XN-Series hematology analyzers in the future.
Learn more at www.sysmex.com/qualitymonitor. Talk with your Sysmex representative for more information.
About Sysmex America, Inc.
Sysmex America, Inc. distributes and supports a diverse portfolio of best-in-class, in vitro diagnostic hematology and clinical laboratory products in the U.S., Canada and Latin America. It is the U.S. headquarters of Sysmex Corporation, located in Kobe, Japan, a trusted world leader in clinical laboratory solutions that optimize efficiency, operations and financial performance in the lab. Sysmex is reshaping the healthcare of tomorrow at the clinical laboratory level by transcending the boundaries of diagnostic science and serving as the pulse of reliable, accountable care. For more information about Sysmex America, visit www.sysmex.com/us.

View original content with multimedia:http://www.prnewswire.com/news-releases/innovation-in-quality-assurance-new-sysmex-beyondcaresm-quality-monitor-300485799.html
SOURCE Sysmex America, Inc.





