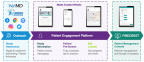
ICON plc announced the release of its web based patient engagement platform, to provide patients with study specific information and connectivity with the nearest investigative site.
Increasing visibility of potential study participants to sites and sponsors
DUBLIN--(BUSINESS WIRE)-- ICON, (NASDAQ: ICLR) a global provider of drug and device development and commercialisation services to the pharmaceutical, biotechnology and medical device industries, today announced the release of its web based patient engagement platform, to provide patients with study specific information and connectivity with the nearest investigative site. The solution supplements patient recruitment outreach by sites and increases visibility of potential study participants for sponsors and sites.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20190509005053/en/

Patient recruitment specialists work with sponsors to develop outreach programs that incorporate the right mix of digital channels, traditional methods and patient advocacy partnerships to attract patients to a study branded website hosted on the platform. An easy to navigate, user friendly interface guides the patient to new and ongoing studies in their particular indication and a pre-qualification questionnaire helps to determine if the study is a right fit for them. If the patient decides to register interest, they are given the option to select their nearest investigative site. This establishes connection with the site and the patient can then choose to contact the site or ask to be contacted for pre-screening.
By being able to access the mobile optimised website at home, patients are able to discuss the possibility of trial participation as a clinical care option with their family and caregivers. Making it easier for the patient to register interest will increase access to potential patients for sponsors. The platform will also enable site staff to see the number of pre-qualification questionnaires completed in near real-time to monitor and report on progress to sponsors.
“Access to patients continues to be the biggest challenge for sponsors, impacting speed to market and overall drug development costs,” commented EB McLindon, Senior VP Site & Patient Solutions, “We have used our understanding of patients gained through managing thousands of trials to develop this patient engagement platform and ease the burden on sites and patients. This will increase the predictability and speed of patient recruitment”.
About ICON plc
ICON plc is a global provider of outsourced drug and device development and commercialisation services to pharmaceutical, biotechnology, medical device and government and public health organisations. The company specialises in the strategic development, management and analysis of programs that support clinical development - from compound selection to Phase I-IV clinical studies. With headquarters in Dublin, Ireland, ICON employed approximately 13,920 employees in 90 locations in 37 countries as at March 31, 2019. Further information is available at www.iconplc.com/patient-platform
ICON/ICLR-G
This press release contains forward-looking statements. These statements are based on management's current expectations and information currently available, including current economic and industry conditions. These statements are not guarantees of future performance or actual results, and actual results, developments and business decisions may differ from those stated in this press release. The forward-looking statements are subject to future events, risks, uncertainties and other factors that could cause actual results to differ materially from those projected in the statements, including, but not limited to, the ability to enter into new contracts, maintain client relationships, manage the opening of new offices and offering of new services, the integration of new business mergers and acquisitions, as well as economic and global market conditions and other risks and uncertainties detailed from time to time in SEC reports filed by ICON, all of which are difficult to predict and some of which are beyond our control. For these reasons, you should not place undue reliance on these forward-looking statements when making investment decisions. The word "expected" and variations of such words and similar expressions are intended to identify forward-looking statements. Forward-looking statements are only as of the date they are made and we do not undertake any obligation to update publicly any forward-looking statement, either as a result of new information, future events or otherwise. More information about the risks and uncertainties relating to these forward-looking statements may be found in SEC reports filed by ICON, including its Form 20-F, F-1, S-8 and F-3, which are available on the SEC's website at http://www.sec.gov.
View source version on businesswire.com: https://www.businesswire.com/news/home/20190509005053/en/
Contacts
ICON Media:
Lucinda Sandon-Allum
Weber Shandwick
+44 (0)2070670548
lsandon-allum@webershandwick.com
Source: ICON plc