Cidara Therapeutics, Inc. (Nasdaq: CDTX), a biotechnology company developing novel anti-infectives including immunotherapies, today announced the presentation of data from the company’s Phase 2 STRIVE trial of rezafungin at IDWeek 2018, taking place in San Francisco from October 3-7.
SAN DIEGO--(BUSINESS WIRE)-- Cidara Therapeutics, Inc. (Nasdaq: CDTX), a biotechnology company developing novel anti-infectives including immunotherapies, today announced the presentation of data from the company’s Phase 2 STRIVE trial of rezafungin at IDWeek 2018, taking place in San Francisco from October 3-7. The STRIVE trial successfully achieved its primary endpoints, demonstrating the efficacy and safety of once-weekly dosing of rezafungin compared to once-daily dosing of caspofungin in patients with candidemia and/or invasive candidiasis. Results from the STRIVE trial will be featured in an oral abstract session along with three additional rezafungin poster presentations which showcase the broad clinical utility of Cidara’s novel once-weekly antifungal agent.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20181004005151/en/
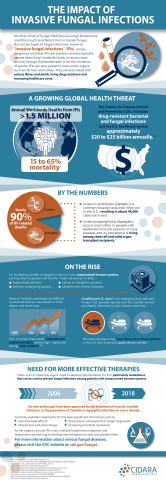
The Impact of Invasive Fungal Infections (Graphic: Business Wire)
“The additional detail from the Phase 2 STRIVE trial bolsters our confidence in achieving the objectives of the recently initiated Phase 3 ReSTORE trial,” said Jeffrey Stein, Ph.D., president and chief executive officer of Cidara. “We believe that rezafungin has the potential to provide an important new treatment option for patients with potentially deadly invasive fungal infections.”
The STRIVE data will be presented by George R. Thompson, M.D., Principal Investigator for the trial, during the oral abstract session, Clinical Trials That May Change Your Practice, on Saturday, October 6, 2018 at 9:30 a.m. Pacific Time
“The data from the Phase 2 STRIVE trial provide evidence of rezafungin’s safety and potent efficacy against Candida infections,” said Dr. Thompson, Associate Professor of Clinical Medicine at the University of California, Davis, School of Medicine and the Departments of Medical Microbiology and Immunology, and Internal Medicine, Division of Infectious Diseases. “The potential of rezafungin to achieve higher drug exposures to treat invasive Candida disease as well as the potential for easy once-weekly outpatient use could be practice-changing in many clinical situations.”
STRIVE was an international, multicenter, double-blind, Phase 2 trial evaluating the safety, tolerability and efficacy of once-weekly dosing of rezafungin acetate compared to once-daily dosing of caspofungin in patients with candidemia and/or invasive candidiasis (IC). STRIVE met all of its primary objectives: once-weekly intravenous (IV) dosing of rezafungin at two dosing regimens was observed to be generally well tolerated and safe in patients with candidemia and/or IC. The data also provide evidence of rezafungin efficacy, which was defined in the trial by clearance of Candida from the blood or other normally sterile sites (mycological response), resolution of signs related to the infection (investigator assessment of clinical response) and overall survival.
Key findings from the three rezafungin IDWeek poster presentations are summarized below.
- Pharmacokinetic-Pharmacodynamic (PK-PD) Target Attainment Analyses to Support Rezafungin (RZF) Dose Selection in Treatment of Candida; E.A. Lakota, et. al.
Using a simulation model, this study evaluated two IV dosing regimens of either 400 mg of rezafungin administered once weekly or 400 mg on Week one, then 200 mg once weekly thereafter. Researchers concluded that rezafungin achieved high target attainment against C. albicans and C. glabrata with both regimens, supporting Phase 3 dose selection for the treatment of patients with candidemia or IC. - Activity of a Long-Acting Echinocandin, Rezafungin, Tested against Invasive Fungal Isolates Collected Worldwide; Mariana Castanheira, PhD, et. al.
The results from this study compared the in vitro activity of rezafungin to other antifungals (e.g., azoles and first-generation echinocandins) against 719 clinical isolates from invasive fungal infections collected worldwide during 2017. The study concluded that rezafungin demonstrated potent activity against this global panel of clinical isolates of Candida and Aspergillus species. The authors noted the potential of rezafungin in prevention and treatment of invasive fungal infections, including in outpatient settings. - Effect of Rezafungin on QT Interval in Healthy Subjects; S. Flanagan, et. al.
This study highlights results from a Phase 1, single-center, randomized, comparative trial of single doses of IV rezafungin, IV placebo, and oral moxifloxacin (positive control) in healthy adult subjects. As some antifungals, such as azoles, are associated with QT prolongation and risk of potentially fatal cardiac irregularities (e.g., torsades de pointes – a serious heart rhythm disturbance), the primary objective was to assess the effects of rezafungin on QT interval. Results showed that rezafungin in single doses up to 1400 mg IV had no significant effect on QT prolongation or on any of the other cardiac conduction parameters tested.
Invasive fungal infections (IFIs) represent a serious threat to millions of patients worldwide resulting in more than 1.5 million deaths annually and mortality rates ranging from 15 to 65 percent. These infections continue to be a global health issue, especially for critically ill patients in hospitals and patients with compromised immune systems, including cancer and transplant patients. Approximately 90 percent of IFI-related hospital deaths in the U.S. are associated with Candida and Aspergillus.
Copies of all IDWeek presentations will be available on the Cidara website following the meeting: www.cidara.com.
About Rezafungin
Rezafungin is a novel antifungal echinocandin being developed as a once-weekly, high-exposure therapy for the treatment and prevention of serious invasive fungal infections. Rezafungin has a unique pharmacokinetic profile with a prolonged half-life and front-loaded plasma exposure which, in contrast to all other echinocandins, allows for once-weekly IV therapy. Rezafungin is being studied to address unmet needs in the treatment of candidemia and invasive candidiasis as well as for prophylaxis (prevention) of invasive fungal infections, including Candida, Aspergillus and Pneumocystis, in patients with hematologic malignancies undergoing allogeneic bone marrow transplantation.
About Cidara Therapeutics
Cidara is a clinical-stage biotechnology company focused on developing new anti-infectives that have the potential to transform the standard of care and save or improve patients’ lives. The company is currently advancing its novel echinocandin antifungal, rezafungin acetate, in a Phase 3 clinical trial in the treatment of candidemia and invasive candidiasis and plans to initiate a second Phase 3 trial in the prophylaxis of invasive fungal infections. Rezafungin has improved pharmacokinetics compared to existing echinocandins and the potential for expanded utility across patient settings. It is the only once-weekly product candidate in development for the treatment and prevention of life-threatening invasive fungal infections. Cidara also is leveraging its novel Cloudbreak™ platform to develop antibody-drug conjugates for the treatment of serious viral and Gram-negative bacterial infections. Cloudbreak is the first immunotherapy discovery platform designed specifically to create compounds that directly kill pathogens and also direct a patient’s immune cells to attack and eliminate bacterial, fungal or viral pathogens. Cidara is headquartered in San Diego, California. For more information, please visit www.cidara.com.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Such statements include, but are not limited to, statements regarding the initiation of rezafungin Phase 3 pivotal trials, the potential for rezafungin to be a novel treatment and prophylactic agent against deadly invasive fungal infections, and represent an improvement over current approaches, rezafungin’s potential for expanded utility across patient settings; and rezafungin’s potential to be practice-changing. Risks that contribute to the uncertain nature of the forward-looking statements include: the success and timing of Cidara’s preclinical studies and clinical trials; regulatory developments in the United States and foreign countries; changes in Cidara’s plans to develop and commercialize its product candidates; Cidara’s ability to obtain additional financing; Cidara’s ability to obtain and maintain intellectual property protection for its product candidates; and the loss of key scientific or management personnel. These and other risks and uncertainties are described more fully in Cidara’s Form 10-Q most recently filed with the United States Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made. Cidara undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.
View source version on businesswire.com: https://www.businesswire.com/news/home/20181004005151/en/
Contacts
Cidara Therapeutics, Inc.
INVESTOR CONTACT:
Westwicke Partners, LLC
Robert H. Uhl
Managing Director
858-356-5932
robert.uhl@westwicke.com
or
MEDIA CONTACT:
Sam Brown Inc.
Christy Curran
615-414-8668
ChristyCurran@sambrown.com
Source: Cidara Therapeutics, Inc.







