- Rimegepant 75 mg, dosed every other day, demonstrates statistically significant superiority, compared to placebo, on the primary endpoint of reduction in the mean number of migraine days per month - Orally administered rimegepant 75 mg, approved earlier this year for the acute treatment of migraine, is the only CGRP targeting therapy to demonstrate efficacy in both the acute and preventive treatment of migraine - Biohaven plans to engage the FDA and European
|
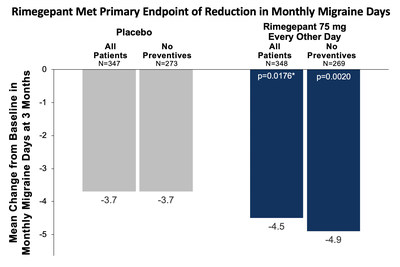
NEW HAVEN, Conn., March 30, 2020 /PRNewswire/ -- Biohaven Pharma Holding Company Ltd. (NYSE: BHVN) today announced positive topline results in its randomized, placebo-controlled pivotal clinical trial (NCT03732638) evaluating the efficacy and safety of oral rimegepant 75 mg for the preventive treatment of migraine in both episodic and chronic migraine patients. The study met the primary endpoint, demonstrating a statistically significant reduction from baseline in monthly migraine days in patients treated with rimegepant compared with placebo. Those receiving rimegepant 75 mg every other day (n=348) experienced a statistically significant 4.5 day reduction from baseline in monthly migraine days, compared to a 3.7 day reduction in the placebo group (n=347; p=0.0176). Among study participants not taking concomitant preventive treatment, there was a 4.9 day reduction in monthly migraine days in the rimegepant group (n=273) compared with a 3.7 day reduction in the placebo group (n=269; nominal p=0.0020). A total of 22% of the study participants were taking a concurrent preventive treatment, including topiramate and amitriptyline. Importantly, 48% of the rimegepant group had at least a 50% reduction from baseline in the mean number of moderate to severe migraine days per month compared to 41% in the placebo group. Vlad Coric, M.D., Chief Executive Officer of Biohaven commented, "Rimegepant is the first CGRP targeting therapy to complete pivotal trials that demonstrate efficacy in both the acute and preventive treatment of migraine. The magnitude of effect in the rimegepant treated arm of a 4.5 day reduction at 3 months in this study was on the higher end of what has been demonstrated by other approved preventive drugs for migraine patients with similar baselines. We believe rimegepant has the potential to change the paradigm of migraine treatment, offering patients the potential for dual-therapy action – acute and preventive treatment — in one simple dose and convenient formulation." Dr. Coric added, "We believe that, for prevention, the ability to take an oral medication every other day rather than recurring injection/intravenous therapy provides patients with a simple, less invasive method of treatment and may allow patients to feel more in control of their migraine. If successful in expanding our label for NURTEC ODT to include prevention, patients for the first time will have the convenience of one oral medication to treat their migraine across the spectrum instead of having to mix different acute and preventive medications." Richard B. Lipton, M.D., Professor and Vice Chair of Neurology at the Albert Einstein College of Medicine and Montefiore Health System, Director of the Montefiore Headache Center commented, "I see many patients who are discouraged by the limited current preventive treatment options and continue to look for a better way to prevent disabling migraine attacks. This is the first time that patients may be able to use a single drug for both acute and preventive treatment. Particularly impressive is the fact that the primary outcome measure of reduction in monthly migraine days was achieved with every other day dosing. I believe that rimegepant will fulfill significant unmet needs for my patients as an oral agent that provides acute and preventive treatment benefits." This pivotal study enrolled patients with both episodic and chronic migraine. The study evaluated the efficacy and safety of rimegepant 75 mg (n=370) dosed every other day for the preventive treatment of migraine versus placebo (n=371) in patients who had migraine for at least one year and 4 to 18 moderate to severe migraine attacks per month over three months prior to enrollment. During the one-month observation period, patients experienced on average 10.7 migraine days during the 4 week month, with 7.4 migraine days of moderate to severe pain intensity migraine during the same period. Robert Croop, M.D., Chief Development Officer – Neurology at Biohaven added, "These data demonstrate rimegepant's broad range of clinical activity to potentially provide a new oral preventive treatment option for people with migraine. The magnitude of effect in the rimegepant treated arm with favorable safety and tolerability suggest that rimegepant could be a best-in class oral therapy for both preventive and acute treatment of migraine. We look forward to sharing detailed results from the study at upcoming medical conferences and continuing to advance this innovative oral treatment for the preventive treatment of migraine." The safety profile seen in the 370 patients who received rimegepant 75 mg every other day was consistent with prior clinical trial experience. There were no cases of ALT or AST > 3 x ULN and bilirubin > 2 x ULN. An independent liver monitoring panel did not determine any ALT/AST elevations to be in the categories of probably or definitely related to study drug. Discontinuation rates were higher in the placebo arm, largely driven by a greater number of placebo patients (6%) withdrawing consent compared to rimegepant treated participants (3%). Additional efficacy and safety study results will be shared at medical conferences later this year. Biohaven Conference Call Information About Rimegepant About Migraine CGRP Receptor Antagonism About NURTEC ODT In a clinical trial, the most common adverse reaction was nausea (2% in patients who received NURTEC ODT compared to 0.4% in patients who received placebo). Hypersensitivity, including dyspnea and rash, occurred in less than 1% of patients treated with NURTEC ODT. For more information about NURTEC ODT, visit www.nurtec.com. About Biohaven Forward-looking Statement Biohaven Contact Media Contact NURTEC and NOJECTION are trademarks of Biohaven Pharmaceutical Holding Company Ltd.
SOURCE Biohaven Pharmaceutical Holding Company Ltd. |
||
Company Codes: NYSE:BHVN |