Baxter International Inc. (NYSE:BAX), a global leader in acute care, announced it has received emergency use authorization (EUA) from the U.S. FDA for the company’s Oxiris filter set to treat patients who have confirmed COVID-19 and have been admitted to the intensive care unit (ICU) with confirmed or imminent respiratory failure in need of blood purification therapy to reduce pro-inflammatory cytokine levels, including use in continuous renal replacement therapy (CRRT).
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20200423005807/en/
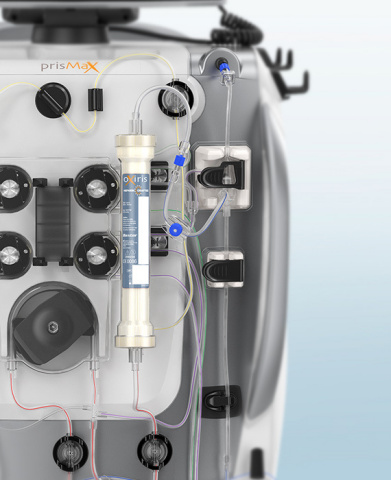
Baxter's Oxiris filter set (Photo: Business Wire)
“We are doing whatever it takes to support healthcare providers as they care for patients during extraordinary circumstances. Oxiris offers a new tool in the COVID-19 fight while supplementing our overall supply of filters for blood purification therapies,” said José (Joe) E. Almeida, chairman and chief executive officer. “We are grateful for the FDA’s collaboration and support to make Oxiris available as quickly as possible to the influx of patients who are critically ill from COVID-19.”
In severe cases of COVID-19, patients may develop acute kidney injury (AKI), a condition where the kidneys suddenly stop working, and/or cytokine storms, which occur when high levels of the inflammatory mediators circulate in the blood as an intense immune reaction to the virus. Both conditions can be life-threatening and require intervention. Early studies suggest that 15 to 30% of patients with severe forms of COVID-19 are developing AKI,1 while 67% of severely ill patients with COVID-19 infection may present with additional organ dysfunction syndromes that could be induced by a high level of circulating cytokines.2
During blood purification therapy, the patient’s blood passes through the Oxiris filter set, where it then removes cytokines, endotoxin, fluid and uremic toxins simultaneously, before returning the patient’s blood to the body. Oxiris is the only filter set available in the U.S. that can be used to perform multiple blood purification therapies simultaneously, including CRRT and cytokine removal. When Oxiris is used, there is no change to traditional CRRT set up and delivery and no additional equipment is required for removal of inflammatory mediators. Unlike other products, Oxiris does not require the use of a second CRRT filter or adsorber, which can help conserve resources.
Oxiris is currently in use across countries in Europe and Asia and has been used for more than 10 years to treat thousands of patients. Oxiris has been validated for use with Baxter’s leading PrisMax and Prismaflex systems. PrisMax, which was launched in the U.S. in 2019, is the company’s next-generation blood purification platform that helps simplify therapy delivery, while providing hospitals the flexibility to meet the unique demands of the ICU.
A small initial shipment of Oxiris will be available in the U.S. immediately, with more significant production ramping up throughout the coming weeks and months.
Increasing Product Supply & Distribution to Respond to COVID-19
Baxter is maximizing production of its CRRT machines, fluids and sets to help address unprecedented surges in demand for its acute dialysis products in Europe and the U.S. The company has added multiple work shifts, with all facilities manufacturing products used in COVID-19 patient care running 24 hours a day, seven days a week. The company is partnering with vendors on a component-by-component basis to procure additional raw materials and parts to support increased production. In addition, Baxter has partnered with its logistics providers to fly critically needed medical devices and medicines back and forth between the U.S. and Europe. Flights started this past weekend, and the company expects the equivalent of one cargo plane per day will be transporting products for the next three weeks. This will accelerate the availability of products for patient care over the second quarter. Read more about our response here.
About Baxter
Every day, millions of patients and caregivers rely on Baxter’s leading portfolio of critical care, nutrition, renal, hospital and surgical products. For more than 85 years, we’ve been operating at the critical intersection where innovations that save and sustain lives meet the healthcare providers that make it happen. With products, technologies and therapies available in more than 100 countries, Baxter’s employees worldwide are now building upon the company’s rich heritage of medical breakthroughs to advance the next generation of transformative healthcare innovations. To learn more, visit www.baxter.com and follow us on Twitter, LinkedIn and Facebook.
Rx Only. For safe and proper use of this device, including contraindications, refer to the full Instructions for Use.
Important Safety Information for Oxiris (oXiris)
The Oxiris Set is indicated for use only with the Prismaflex or PrisMax control unit.
The Oxiris Set is authorized by FDA under an Emergency Use Authorization (EUA) to treat patients with COVID-19 infection. Under the terms of the EUA, it is intended to treat patients 18 years of age or older with confirmed COVID-19 admitted to the ICU with confirmed or imminent respiratory failure in need of blood purification, including use in continuous renal replacement therapy, to reduce pro-inflammatory cytokine levels, who have any one of the following conditions:
- Early acute lung injury (ALI)/ early acute respiratory distress syndrome (ARDS);
- Severe disease, such as:
- dyspnea,
- respiratory frequency ≥ 30/min,
- blood oxygen saturation ≤ 93%,
- partial pressure of arterial oxygen to fraction of inspired oxygen ratio < 300,and/or
- lung infiltrates >50% within 24 to 48 hours; or
- Life-threatening disease, defined as:
- respiratory failure,
- septic shock, and/or
- multiple organ dysfunction or failure
Important Safety Information for PrisMax and Prismaflex
The PrisMax and Prismaflex systems are intended for:
CRRT for patients weighing 20 kg or more with acute renal failure and/or fluid overload.
TPE therapy for patients weighing 20 kg or more with diseases where removal of plasma components is indicated.
All treatments administered via the PrisMax and Prismaflex control units must be prescribed by a physician.
This release includes forward-looking statements concerning Oxiris, PrisMax and Prismaflex, including potential benefits associated with their use, and the company’s response to the COVID-19 epidemic, including with respect to the company’s ability to support heightened product demand levels (through the new airbridge or otherwise) and to make product allocations based on need and its plans to hire additional employees. The statements are based on assumptions about many important factors, including the following, which could cause actual results to differ materially from those in the forward-looking statements: ability to maintain supply continuity; actions of regulatory bodies and other governmental authorities; contractual requirements, product quality, manufacturing or supply, or patient safety issues; changes in law and regulations; and other risks identified in Baxter's most recent filing on Form 10-K and other SEC filings, all of which are available on Baxter's website. Baxter does not undertake to update its forward-looking statements.
Baxter, PrisMax, Prismaflex and Oxiris are registered trademarks of Baxter International Inc.
1 Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study [published online ahead of print, 2020 Feb 24] [published correction appears in Lancet Respir Med. 2020 Apr;8(4):e26]. Lancet Respir Med. 2020;. doi:10.1016/S2213-2600(20)30079-5; Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30;:]. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5; Naicker S, Yang CW, Hwang SJ, Liu BC, Chen JH, Jha V. The Novel Coronavirus 2019 epidemic and kidneys [published online ahead of print, 2020 Mar 7]. Kidney Int. 2020;. doi:10.1016/j.kint.2020.03.001
2 Ronco C, Reis T, De Rosa S. Coronavirus epidemic and extracorporeal therapies in intensive care: si vis pacem para bellum [published online ahead of print, 2020 Mar 13]. Blood Purif. 2020;1–4. doi:10.1159/000507039
View source version on businesswire.com: https://www.businesswire.com/news/home/20200423005807/en/
Contacts
Media Contact
Lauren Russ, (224) 948-5353
media@baxter.com
Investor Contact
Clare Trachtman, (224) 948-3020
Source: Baxter International Inc.







