BOSTON, MA and SAN ANTONIO, TX--(Marketwired - February 26, 2015) -
| Highlighted Links |
Intrinsic Imaging home page |
Intrinsic Imaging Core Lab Services |
Intrinsic Imaging offers Sponsors a wealth of therapeutic and operational expertise to best optimize the management and analysis of medical images in support of Phase I, II and III clinical trials in support of New Drug Applications, and in Class I, II and III Medical Device clinical trials in support of 510(k) clearances and Premarket Approvals (PMA).
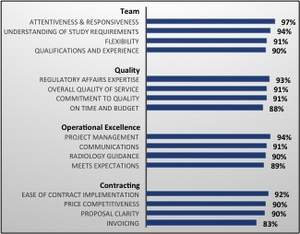
Intrinsic Imaging annually sets and measures its performance versus predefined quality objectives. For the fiscal year ending December 31st, 2014, all customers were asked to rate Intrinsic Imaging's performance versus 16 predefined quality objectives, categorized into groups, including Team, Quality, Operational Excellence, and Contracting.
In their evaluation of Intrinsic Imaging's team, Sponsors unanimously reported that they are exceptionally satisfied with Intrinsic Imaging's qualifications and experience (with an average score of 90%), flexibility (91%), understanding of study requirements (94%) and overall attentiveness and responsiveness (97%). The high degree of customer satisfaction is due in large part to Intrinsic Imaging's experienced project management team as well as their 65 full-time, board-certified, fellowship-trained radiologists, who bring extensive experience and specialization in all therapeutic areas including, but not limited to, Cardiovascular, Central Nervous System, Gastrointestinal & Genitourinary, Medical Device, Musculoskeletal and Oncology.
In rating their satisfaction with Intrinsic Imaging's quality, Sponsors recognized the company's expertise and universally reported their satisfaction with its ability to deliver quality data on time and on budget (88%), its commitment to quality (91%) as well as the company's overall quality of service (91%) and in-depth understanding of the regulatory requirements (93%).
"Intrinsic Therapeutics is a medical device company dedicated to the responsible development and commercialization of medical devices to treat lumbar disc herniations. In selecting Intrinsic Imaging as our imaging core lab partner, it was essential that they shared our commitment to high quality standards," said Jacob Einhorn, Chief Operating Officer, Intrinsic Therapeutics. "Intrinsic Imaging has continuously proven to us that they have an in-depth understanding of medical devices, are dedicated to quality, and have the project management expertise, technology and skillset to manage medical device clinical trials effectively."
Sponsors also recognized Intrinsic Imaging's expertise and rated exceptional satisfaction with the company's operational excellence, scoring its project management skills at 94 percent, its open, honest and clear communications at 91 percent, its effectiveness of providing radiology guidance at 90 percent and the company's overall ability to meet client expectations at 89 percent. Administratively, Intrinsic Imaging also scored exceptionally well in the area of contracting. Its ease of implementation, competitive pricing, proposal clarity and invoicing all received high levels of satisfaction.
"Intrinsic Imaging has an unwavering commitment to quality. It is important to us that we are cognizant of our customers' satisfaction with our performance in managing their clinical trials, so we can ensure we are always meeting or surpassing expectations," said Todd Joron, BSc., MBA, President & Chief Operating Officer. "We are proud that the results of this year's survey confirm that our customers are exceedingly satisfied with their experience working with Intrinsic Imaging. Our in-depth radiology expertise across all therapeutic areas, scalable technologies and ISO certified and registered quality management systems allow us to provide unprecedented services to Sponsors."
About Intrinsic Imaging
Located in Bolton, Massachusetts and San Antonio, Texas, Intrinsic Imaging is an FDA audited, ISO 9001:2008 and ISO 13485:2003 certified, GAMP® 5 compliant medical imaging core lab specializing in providing imaging core lab services for clinical trials. Its comprehensive medical imaging core lab services include, but are not limited to, expert radiologist consultation, protocol and charter development, site qualification, training and management, as well as image acquisition, processing and detailed radiologic analysis.
Intrinsic Imaging has more than sixty full-time, board-certified diagnostic radiologists on staff that have sub-specialization in all therapeutic areas including, but not limited to, Cardiovascular, Central Nervous System, Gastrointestinal & Genitourinary, Musculoskeletal, Medical Device, and Oncology.
Help employers find you! Check out all the jobs and post your resume.