When presented with ORLADEYO, both patients and physicians recognized immediately the freedom gained with the once-daily prophylactic therapy and indicated a high willingness to use.
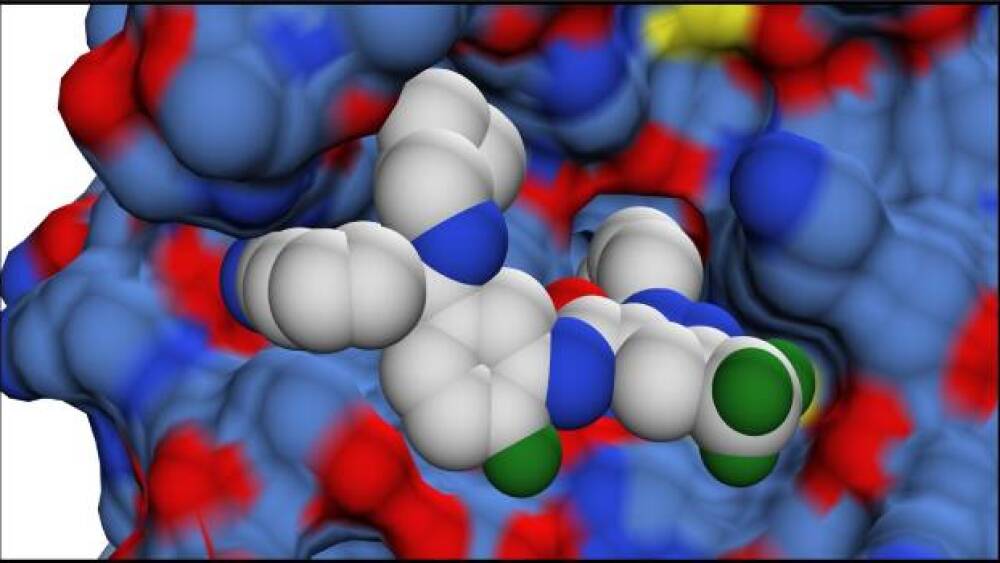
3D rendering of the molecular structure of ORLADEYO® (berotralstat) bound to the active site of Human Plasma Kallikrein/Courtesy BioCryst
One year ago, with pandemic lockdowns in full force, BioCryst launched ORLADEYO® (berotralstat), an oral, once-daily prophylactic drug for hereditary angioedema (HAE) in patients 12 years of age and older. Safety and effectiveness of ORLADEYO for the treatment of acute HAE attacks have not been established.
A “pandemic launch” in itself was uncharted territory, but what makes the story even more interesting is the fact that patients and physicians said they were already very satisfied with their existing HAE medications. On paper, this launch appeared to be an uphill battle for BioCryst.
HAE is a rare genetic disease characterized by severe swelling in parts of the body – including the face or throat. In those instances, it can be fatal.
During the past 15 years, “There has been steady movement towards preventing attacks versus just treating them if they happen,” Charlie Gayer, chief commercial officer, told BioSpace. For example, patients went from twice-weekly infusions to self-administered subcutaneous injections every three or four days, to subcutaneous injections every two weeks. “These drugs change people’s lives for the better,” Gayer said. But, “As good as they are, they come with a significant burden.”
As he explained, intravenous infusions are administered twice weekly. That may require patients to visit their doctors twice each week, or for nurses to visit their homes, but they can also self-administer. Subcutaneous injections relieve that burden, but some require refrigeration, so patients who travel must carry cold packs to protect their medication.
“When we diligently gathered insights from these patients, we learned that they were, understandably, grateful and satisfied with every advancement in the treatment of their HAE,” said Jinky Rosselli, BioCryst’s chief data and insights officer. “This naturally led us to the next question, ‘were there any remaining unmet needs left in the HAE market, given this level of satisfaction?’”
Rosselli explained further, “Our team mined the insights carefully and discovered the true psychology driving the high satisfaction. People living with HAE were expert-copers, not readily recognizing the burden of these treatments, mainly because there were no other options available.”
According to Rosselli, when presented with ORLADEYO, both patients and physicians recognized immediately the freedom gained with the once-daily prophylactic therapy and indicated a high willingness to use. “We saw this strong interest demonstrated in our research, and we see it even more pronouncedly now in the marketplace as more patients use ORLADEYO.”
BioCryst’s ethnographic studies showed, “Typically, physicians focus their conversations with patients on their last HAE attack,” Gayer explained. “However, when physicians evolve their discussion and ask the patient about their lives overall, and ask about the impact of their current treatment on their daily living, patients and physicians then both see why switching from an injection or infusion to a pill can be beneficial.”
BioCryst’s ORLADEYO is an oral, once-daily pill. ORLADEYO, a small molecule, inhibits kallikrein (an enzyme in the inflammation cascade). It fits into the active site of the kallikrein protein and blocks its activation so the kallikrein can’t cleave and activate bradykinin – an inflammatory molecule produced in excess in HAE patients. This is designed to prevent HAE attacks.
Oral administration allows patients to live without the burdens of injectable medications, but studies also showed that ORLADEYO is effective.
Data were presented at the European Academy of Allergy and Clinical Immunology (EAACI) Congress in July 2021. An ad hoc analysis of interim data showed that the 21 patients who were taking ORLADEYO 150 mg achieved an 86% reduction from baseline at week 96 in the average rate of attacks they experienced. “That’s important,” Gayer said. “It means patients went from an average of approximately three attacks per month to an average of about half an attack per month.”
Additional data presented at the American College of Asthma, Allergy and Clinical Immunology (ACAAI) in November examined patients in the United States who switched from injectable prophylaxis to ORLADEYO during the APeX-S long-term safety study. Regardless of which therapy patients switched from or when they switched, ORLADEYO provided consistently low attack rates when used as a preventive monotherapy in these patients.
The safety findings of APeX-S were similar to previous clinical studies and in line with the known safety profile of ORLADEYO. The most common adverse reactions in patients receiving ORLADEYO are abdominal pain, vomiting, diarrhea, back pain and gastroesophageal reflux disease.
Switching from another HAE drug to ORLADEYO should be based on a patient’s clinical condition and their physician’s discretion, Gayer said. “For the C1 inhibitor drugs, based on the half-life, there would be a two-week overlap for ORLADEYO to reach a steady state. A subcutaneous injection dosed every two weeks doesn’t require an overlap period,” he said, noting that stress is a trigger for HAE attacks.
Despite launching ORLADEYO during the height of the pandemic, “In some ways, the timing was fortunate,” Gayer said. “We had a plan to hire the sales team and to have the sales reps in place six months before the December launch. By April 2020, it was clear the lockdown wouldn’t be a two- to four-week thing, so we hired our entire sales team virtually.”
By the time of the launch, the reps knew their key targets, understood their practices and how they worked and had virtual visits to validate and prioritize the physicians. “That helped us get out of the gate fast,” Gayer said.
“We also were fortunate that physicians treating HAE weren’t as affected by COVID-19 as some other specialties,” Rosselli said. “Physicians were still getting up to speed (on telemedicine) and learning the best ways to ‘see’ their patients and optimizing their patient communication platforms. We quickly implemented the best tools available and trained reps on the best ways to engage and educate physicians on ORLADEYO.”
Ensuring patients had access to ORLADEYO was also paramount. Gayer said, “In order to find the most efficient way for patients to receive ORLADEYO, we chose a sole-source specialty pharmacy with proven experience in rare disease therapies, rather than creating a patient hub with multiple pharmacies. We streamlined the entire process by simplifying forms, establishing dedicated care coordinators for patients and physicians to help with any paperwork and approvals needed for ORLADEYO. As a result, patient access is intended to be simpler and more efficient than traditional specialty pharmacies.
That approach contributed to its solid sales. ORLADEYO is approved for sale in the U.S., EU, U.K., Japan and United Arab Emirates. In the first year of its launch, ORLADEYO has generated approximately $122 million in net revenue.
U.S. Indication and Important Safety Information
INDICATION
ORLADEYO® (berotralstat) is a plasma kallikrein inhibitor indicated for prophylaxis to prevent attacks of hereditary angioedema (HAE) in adults and pediatric patients 12 years and older.
Limitations of use
The safety and effectiveness of ORLADEYO for the treatment of acute HAE attacks have not been established. ORLADEYO should not be used for the treatment of acute HAE attacks. Additional doses or dosages of ORLADEYO higher than 150 mg once daily are not recommended due to the potential for QT prolongation.
IMPORTANT SAFETY INFORMATION
An increase in QT prolongation was observed at dosages higher than the recommended 150 mg once-daily dosage and was concentration dependent.
The most common adverse reactions (≥10 percent and higher than placebo) in patients receiving ORLADEYO were abdominal pain, vomiting, diarrhea, back pain, and gastroesophageal reflux disease.
A reduced dosage of 110 mg taken orally once daily with food is recommended in patients with moderate or severe hepatic impairment (Child-Pugh B or C) and in patients taking chronically administered P-glycoprotein (P-gp) or breast cancer resistance protein (BCRP) inhibitors (eg, cyclosporine).
Berotralstat is a substrate of P-gp and BCRP. P-gp inducers (eg, rifampin, St. John’s wort) may decrease berotralstat plasma concentration, leading to reduced efficacy of ORLADEYO. The use of P-gp inducers is not recommended with ORLADEYO.
ORLADEYO at a dose of 150 mg is a moderate inhibitor of CYP2D6 and CYP3A4. For concomitant medications with a narrow therapeutic index that are predominantly metabolized by CYP2D6 or CYP3A4, appropriate monitoring and dose titration is recommended. ORLADEYO at a dose of 300 mg is a P-gp inhibitor. Appropriate monitoring and dose titration is recommended for P-gp substrates (eg, digoxin) when coadministering with ORLADEYO.
The safety and effectiveness of ORLADEYO in pediatric patients <12 years of age have not been established.
There are insufficient data available to inform drug-related risks with ORLADEYO use in pregnancy. There are no data on the presence of berotralstat in human milk, its effects on the breastfed infant, or its effects on milk production.
To report SUSPECTED ADVERSE REACTIONS, contact BioCryst Pharmaceuticals, Inc. at 1-833-633-2279 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information.