SonaCare Medical's CSO, Naren Sanghvi, Recognized by Prestigious Focused Ultrasound Foundation with 2018 Visionary Award
CHARLOTTE, N.C., Oct. 25, 2018 /PRNewswire/ -- SonaCare Medical, the leading developer and manufacturer of high intensity focused ultrasound (HIFU) technologies, congratulates Naren Sanghvi on his receipt of the Focused Ultrasound Foundation's 2018 Visionary Award, which recognizes his crucial role in advancing the field of focused ultrasound. This award is given every two years and recognizes an individual who has created a larger vision for what the future of focused ultrasound may hold and whose effort, passion, and persistence have been crucial to advancing the field.
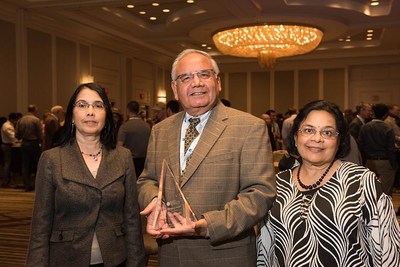
The mission of the Focused Ultrasound Foundation is to accelerate the development and adoption of focused ultrasound technology as a standard of care. Naren Sanghvi has lived this mission for more than 45 years, dating back to early in his career working with focused ultrasound in the Fry brothers' laboratory at Indiana University School of Medicine. Naren's acknowledgement by the leading society in the field punctuates the technological leadership SonaCare Medical HIFU technology has had in patient care over the past 15 years. As the inventor and developer of the Sonablate® HIFU prostate tissue ablation device, Naren's work has touched the lives of over 20,000 patients with prostate cancer. He is not done yet; seminal work he has done in the development of additional applications of SonaCare technology will be introduced clinically over the next 18 months.
Dr. John Juride, urologic surgeon and longtime Sonablate user, comments: "Naren Sanghvi is a true pioneer in the field of HIFU. Clinicians and patients around the world are indebted to him for his many contributions to this remarkable technology."
Dr. Sanghvi currently is Chief Scientific Officer at SonaCare Medical. Since the Company's inception, he has played a critical role in developing Sonablate® technology to be the most advanced minimally invasive surgical robotic ultrasound system for prostate tissue ablation in the world. Naren's contributions have made SonaCare Medical both an industry and technology leader in the field of focused ultrasound ablation.
"We are proud to have Naren Sanghvi recognized by the Focused Ultrasound Foundation," comments SonaCare Medical CEO, Dr. Mark Carol. "This award could not be given to a more deserving individual. Naren has been, and continues to be, a cornerstone in technology leadership at SonaCare Medical. He is known, recognized, and beloved by all who work in the field. He continues to forge a path that will influence focused ultrasound technology, and the patients it is used to treat, for many years to come."
Since Sonablate® received FDA clearance on October 09, 2015, more than 2,100 patients have had a Sonablate HIFU prostate procedure across the 70+ locations in the U.S., including top-tier academic institutions in California, Indiana, Oklahoma, Maryland, New York, Arizona, and Texas. Over 70 U.S. physicians now offer HIFU prostate tissue ablation to their patients as a minimally invasive alternative to surgery or radiation, a number that is growing monthly. In addition, Sonablate HIFU prostate treatments are available in more than 50 countries worldwide where Sonablate has been used for close to 20 years.
ABOUT SONACARE MEDICAL, LLC
SonaCare Medical is a world leader in minimally invasive focused ultrasound technologies. SonaCare Medical is committed to developing focused ultrasound related technologies that support precise and innovative procedures for the treatment of a range of medical conditions. SonaCare Medical, with its subsidiary Focus Surgery, Inc., designs and manufactures medical devices, including the following: Sonablate®, which has 510(K) clearance in the U.S.; Sonablate® 500, which has CE Marking and has obtained regulatory authorization in more than 50 countries outside the U.S.; Sonatherm® laparoscopic HIFU surgical ablation system, which has 510(K) clearance in the U.S., has CE Marking and has obtained regulatory authorization in more than 30 countries outside the U.S.
For additional information, visit www.SonaCareMedical.com
FORWARD LOOKING STATEMENTS
The Company's forward-looking statements are based on management's current expectations and assumptions regarding the Company's business and performance, the economy and other future conditions and forecasts of future events, circumstances and results. As with any projection or forecast, forward-looking statements are inherently susceptible to uncertainty and changes in circumstances. The Company's actual results may vary materially from those expressed or implied in its forward-looking statements. Any forward-looking statement made by the Company speaks only as of the date on which it is made. The Company is under no obligation to, and expressly disclaims any obligation to, update or alter its forward-looking statements, whether as a result of new information, subsequent events or other factors.
Related Links
http://www.SonaCareMedical.com

![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/sonacare-medicals-cso-naren-sanghvi-recognized-by-prestigious-focused-ultrasound-foundation-with-2018-visionary-award-300737463.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/sonacare-medicals-cso-naren-sanghvi-recognized-by-prestigious-focused-ultrasound-foundation-with-2018-visionary-award-300737463.html
SOURCE SonaCare Medical
