Recce Pharmaceuticals Announces Positive Data on RECCE® 327 Against Influenza A Respiratory Virus Infection in Animal Model
Highlights:
- Dramatic reduction in viral load in the lungs of mice treated with RECCE® 327 as compared to the approved antiviral drug treated and vehicle control untreated groups
- RECCE® 327’s unique mechanism of action reinforces efficacy against both bacterial cells and enveloped viruses
SYDNEY, Australia, April 20, 2020 (GLOBE NEWSWIRE) -- Recce Pharma Ltd (ASX: RCE), the company developing a new class of broad-spectrum synthetic antibiotics, today announced positive efficacy data showing significant in vivo anti-viral activity against the Influenza A virus in mice treated with its lead compound RECCE® 327.
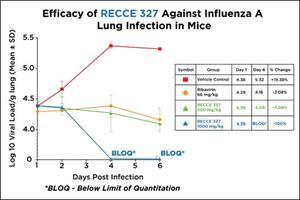
Data showed a significant dose-dependent decrease in the viral growth rate and viral load in lungs of mice infected with Influenza A following treatment with RECCE® 327 compared to the vehicle control group, and group treated with an approved antiviral drug ribavirin, also known as tribavirin.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/c988a256-3ccb-4275-926b-fa0f50f3ba31
| Day | Vehicle control | Ribavirin 66 mg/kg i.p. | RECCE® 327 500 mg/kg iv bid | RECCE® 327 1000 mg/kg iv bid | ||||||||
| Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | N | |
| 1 | 4.38 | 0.1 | 3 | 4.29 | 0.06 | 3 | 4.39 | 0.09 | 3 | 4.39 | 0.1 | 3 |
| 2 | 4.66 | 0.1 | 3 | 4.31 | 0.08 | 3 | 4.35 | 0.03 | 3 | 4.37 | 0.1 | 3 |
| 4 | 5.37 | 0 | 3 | 4.38 | 0.18 | 3 | 4.27 | 0.03 | 3 | BLOQ | NA | 3 |
| 6 | 5.32 | NA | 1 | 4.16 | 0.17 | 3 | 4.09 | 0.02 | 3 | BLOQ | NA | 3 |
NA – Not Applicable
BLOQ – Below Limit of Quantitation
The study was conducted by an independent Contract Research Organization to assess the dose-dependent efficacy of RECCE® 327 and in vivo anti-viral activity against Influenza A, a respiratory lung infection, in mice. RECCE® 327 treatment groups were dosed twice-daily for five days and the ribavirin treatment group was dosed once daily for five days (optimized dosing for latter). Viral pathogenesis in the mice was evaluated by body weight loss, survival and viral load in lungs.
The results demonstrated RECCE® 327 showed a significant dose-dependent decrease in viral load in the lungs compared to the vehicle control and ribavirin – a drug that decreases relapse rates by accelerating viral clearance early in the treatment course1.
Four groups of 12 mice infected with Influenza A showed those treated with RECCE® 327 fared better in almost all instances than those who received the ribavirin treatment, an approved antiviral drug, or vehicle control group. RECCE® 327 continued to show efficacy at different dose levels with significant reduction in viral count in the lungs when compared with the vehicle control. As dosage increased from 500mg/kg to 1,000mg/kg, the viral count fell below the limit of quantitation (BLOQ) on days four and six post-infection.
Influenza A viruses are enveloped viruses and causative agents of respiratory disease. The genome of the Influenza A viruses comprises single-stranded ribonucleic acid (RNA) molecules – similar to that of coronaviruses, where the genome also comprises single-stranded RNA.
Michele Dilizia, Executive Director of Regulatory Affairs, said, “These data reinforce RECCE® 327’s unique, universal mechanism of action, seen against bacterial cells and further now, viral particles. The universal mechanism of action shows continued potency against mutating bacterial cells and it is exciting to see the potential here as well, against viral particles, which are also notorious for their mutation.”
Dr John Prendergast, Non-Executive Chairman said, “The increasing potential to be effective against not only a broad range of superbug bacteria, but viral pathogens as well, reinforces the company’s expanding infectious disease capabilities and global positioning.”
1 Drug Bank CA - https://www.drugbank.ca/drugs/DB00811
This announcement has been approved for release by Recce Pharmaceuticals’ Executive Director James Graham
About Recce Pharmaceuticals Ltd
Recce Pharmaceuticals Ltd (ASX: RCE) is pioneering the development and commercialization of a New Class of Synthetic Antibiotics with Broad Spectrum activity designed to address the urgent global health problem of antibiotic resistant superbugs.
Recce antibiotics are unique – their potency does not diminish even with repeated use, which is a common failure associated with existing antibiotic use and the resulting emergence of resistant superbugs.
Patented lead candidate RECCE® 327, wholly owned and manufactured in Australia, has been developed for the treatment of blood infections and sepsis derived from E. coli and S. aureus bacteria– including their superbug forms.
The FDA has awarded RECCE® 327 Qualified Infectious Disease Product designation under the Generating Antibiotic Initiatives Now (GAIN) Act – labelling it for Fast Track Designation, plus 10 years of market exclusivity post approval.
Recce wholly owns its automated manufacturing, ready to support first-in-human clinical trials. Recce’s anti-infective pipeline seeks to exploit the unique capabilities of RECCE® technologies targeting synergistic, unmet medical needs.
| Executive Director | Media & Investor Relations (AU) | Media & Investor Relations (USA) |
| James Graham | Andrew Geddes | Meredith Sosulski, PhD |
| Recce Pharmaceuticals, Ltd. | CityPR | LifeSci Communications |
| +61 (02) 8075 4585 | +61 (02) 9267 4511 | +1 929 469 3851 |
| james.graham@recce.com.au | ageddes@citypublicrelations.com.au | msosulski@lifescicomms.com |