Recce Pharmaceuticals Announces Lead Synthetic Anti-infective RECCE® 327 Demonstrates Outstanding Efficacy Against Necrotizing Fasciitis ‘Flesh-Eating’ Bacteria
Highlights
- RECCE® 327 (R327) shown to reduce deadly ‘flesh-eating’ bacterial count Below Limit of Quantification (BLOQ) within 24 hours, at varying concentrations
- R327 BLOQ efficacy as early as 30 minutes in C. perfringens – a leading bacterial cause of myonecrosis (gas gangrene)1
- 99.9% (3-log) bacterial reduction achieved in all bacteria tested, at various concentrations
- Data demonstrates R327’s potential against bacterial infections that thrive in nil/low oxygen environments i.e. diabetic wounds/ulcers infections
SYDNEY, Australia, July 20, 2021 (GLOBE NEWSWIRE) -- Recce Pharma Ltd (ASX:RCE) (FSE:R9Q) (Company), the Company developing new classes of synthetic anti-infectives, is pleased to announce positive efficacy of RECCE® 327 (R327) against Clostridium perfringens (C. perfringens) and Streptococcus pyogenes (S. pyogenes), two main strains of bacteria associated with necrotizing fasciitis, also known as ‘flesh-eating’ disease – a life-threatening bacterial infection with a mortality rate of up to 80%.2
Chief Scientific Officer, Michele Diliza, said, “While necrotizing fasciitis is a rare and extremely challenging disease for physicians to manage, it is highly traumatizing for patients and their families, often leading to serious complications and even death. A broad spectrum anti-infective with rapid efficacy has the potential to significantly change the treatment paradigm and save lives. We have been thoroughly impressed with the efficacy that R327 has demonstrated thus far, as it reinforces our belief in the potential of this compound against such aggressive, life-threatening bacteria.”
The study was conducted by an independent contract research organization to evaluate the in vitro bactericidal properties of R327 against C. perfringens and S. pyogenes. Doses of R327 up to 4,800 parts per million (ppm) were tested, covering concentrations from 0.5x to 8x the minimum inhibitory concentration (MIC). The strains tested were: S.pyogenes – a susceptible strain; S.pyogenes – an erythromycin-resistant strain; and C.perfringens.
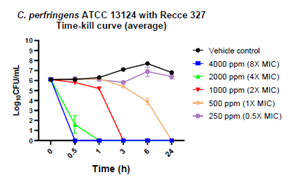
Figure 1: Bactericidal activity of R327 on C. perfringens:
https://www.globenewswire.com/NewsRoom/AttachmentNg/40aba38d-b7fe-4969-bc7e-9e1546fbd28d
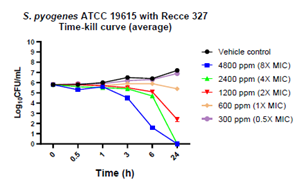
Figure 2: Bactericidal activity of R327 on S. pyogenes ATCC 19615:
https://www.globenewswire.com/NewsRoom/AttachmentNg/e2662c7a-af73-438b-a7da-46d8dab047c7
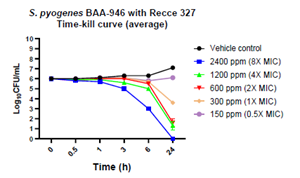
Figure 3: Bactericidal activity of R327 on erythromycin-resistant S. pyogenes BAA-946:
https://www.globenewswire.com/NewsRoom/AttachmentNg/106333ea-75aa-4ae8-a91f-63d680e7e0ec
Time-kill summary, Time (hrs) with a 99.9% (3-log reduction) reduced bacterial count
| Compound | Test Strain | Test Concentration (ppm) |
Time of 3-log reduction (hrs) |
BLOQ* (24hr count) |
|
| R327 | S. pyogenes - susceptible strain | 4800 (8x MIC) | 6 to 24 | ✓ | |
| 2400 (4x MIC) | 24 | ✓ | |||
| 1200 (2x MIC) | 24 | - | |||
| S. pyogenes - erythromycin-resistant strain | 2400 (8x MIC) | 6 to 24 | ✓ | ||
| 1200 (4x MIC) | 24 | - | |||
| 600 (2x MIC) | 24 | - | |||
| C. perfringens | 4000 (8x MIC) | 0.5 to 24 | ✓ | ||
| 2000 (4x MIC) | 0.5 to 24 | ✓ | |||
| 1000 (2x MIC) | 3 to 24 | ✓ | |||
| 500 (1x MIC) | 24 | ✓ | |||
Time of the 3-log10 reduction is the time point in which CFU counts at the respective test condition is reduced relative to the initial CFU counts by ≥3-log10. The initial CFU counts are at the start, time 0, of the assay.
*BLOQ – Below Limit of Quantification
The R327 concentrations and time points that yielded a bactericidal effect are as follows:
- At hours 6 and 24, R327 showed bacteridical activity against the S. pyogenes susceptible strain at 1,200 ppm, 2,400 ppm and 4,800 ppm, while also achieving aBLOQ at the 24 hour count with concentrations of 2,400 ppm and 4,800 ppm (4X – 8X MIC).
- In addition to the susceptible strain, R327 achieved bactericidal effect against the S. pyogenes erythromycin-resistant strain at concentrations 600, 1,200 ppm and 2,400 ppm (2X – 8X MIC), while achieving a BLOQ at 2,400 ppm (8X MIC) in 24 hour counts.
- R327 further demonstrated bactericidal effect against C. perfringens at 500 ppm, 1,000 ppm, 2,000 ppm and 4,000 ppm (1X – 8X MIC) respectively, showing efficacy as early as 30 minutes, and a BLOQ at these concentrations in 24 hour counts.
S. pyogenes is a species of Gram-positive bacteria and can cause life-threatening infections such as scarlet fever, bacteremia (bacteria in the blood), pneumonia, necrotizing fasciitis, myonecrosis and streptococcal toxic shock syndrome. C. perfringens is also a Gram-positive pathogen that is a leading cause of myonecrosis and a species that thrives in nil/low oxygen environments, which can cause infections in diabetic wounds/ulcers – a common characteristic of bacterial anaerobes.
¹ Stevens, D. L., Aldape, M. J., & Bryant, A. E. (2012). Life-threatening clostridial infections. Anaerobe, 18(2), 254-259.
² https://www.ncbi.nlm.nih.gov/books/NBK430756/
About Recce Pharmaceuticals Ltd
Recce Pharmaceuticals Ltd (ASX: RCE) is pioneering the development and commercialisation of New Classes of Synthetic Anti-Infectives designed to address the urgent global health problems of antibiotic resistant superbugs and emerging viral pathogens.
Recce’s anti-infective pipeline is unique and comprised of broad-spectrum synthetic polymer antibiotics RECCE® 327, RECCE® 435, and RECCE® 529 for viral infections with unique mechanisms of action against hyper-mutation on bacteria and viruses, respectively.
Patented lead candidate RECCE® 327 as an intravenous therapy, is being developed for treatment of serious and potentially life-threatening infections including sepsis due to Gram-positive and Gram-negative bacteria including their superbug forms. Recce’s new antibiotic compound, RECCE® 435, has been formulated for oral use.
The FDA has awarded RECCE® 327 Qualified Infectious Disease Product designation under the Generating Antibiotic Initiatives Now (GAIN) Act – labelling it for Fast Track Designation, plus 10 years of market exclusivity post approval. Further to this designation, RECCE® 327 has been included on The Pew Charitable Trusts Global New Antibiotics in Development Pipeline as the only synthetic polymer and sepsis drug candidate in development.
Recce wholly owns its automated manufacturing, ready to support first-in-human clinical trials. Recce’s anti-infective pipeline seeks to exploit the unique capabilities of RECCE® technologies targeting synergistic, unmet medical needs.
Corporate Contact
James Graham
Recce Pharmaceuticals Ltd
+61 (02) 8075 4585
James.graham@recce.com.au
Media and Investor Relations (AU)
Andrew Geddes
CityPR
+61 (02) 9267 4511
ageddes@citypublicrelations.com.au
Media and Investor Relations (USA)
Jordyn Temperato
LifeSci Communications
jtemperato@lifescicomms.com