Novel Packaging and Expression Plasmids Promise to Reduce Gene Therapy Manufacturing Costs and Delivery Times
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20190123005452/en/
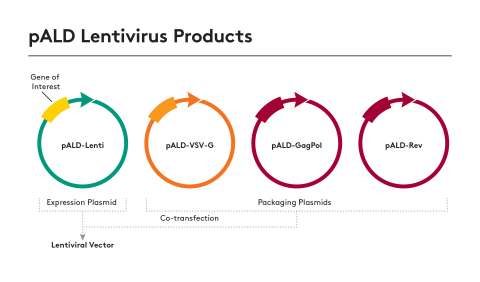
Aldevron's pALD Lenti product line of packaging and expression plasmids offers the highest quality, optimized for any recombinant lentiviral vector production. (Graphic: Aldevron)
The pALD Lenti product line of pALD-Rev, pALD-VSV-G and pALD-GagPol packaging plasmids will be immediately available for research applications, with GMP-Source™ availability beginning in summer of 2019. Clients will be able to provide their gene of interest and Aldevron will clone the construct into the lentivirus expression plasmid for custom production. Together these four plasmids can be used for recombinant lentiviral vector production, thereby allowing all researchers to benefit from shared large-scale production and fast delivery times.
“Genetic medicine has experienced tremendous growth with the approval of multiple products that dramatically improve life,” said Michael Chambers, Aldevron’s CEO. “Lentiviral vectors are the key component for manufacturing many of these treatments. The free availability of lentiviral packaging plasmids will significantly reduce the time and cost to develop and commercialize these products.”
Chambers added that Aldevron’s production scale and experience with custom manufacturing of lentiviral expression plasmids combined with the in-stock packaging plasmids provide clients with a complete set of products and services for their programs. “The lack of complicated intellectual property and royalty structures will further enable our clients and the patients they serve,” added Chambers.
“It is a pleasure to be working with a market-leading group like Aldevron. From our early discussions, Oxford Genetics felt that there was a strong alignment of our individual strengths,” said Ryan Cawood, CEO of Oxford Genetics. “This partnership with Aldevron will serve the needs of the gene and cell therapy industry by improving access to Oxford Genetics’ proven next-generation lentiviral plasmid system.”
To request more information on Aldevron’s lentivirus products, please visit the pALD Lenti product page.
About Aldevron
Aldevron serves the biotechnology industry with custom production of nucleic acids, proteins, and antibodies. Thousands of clients use Aldevron-produced plasmids, RNA and gene editing enzymes for projects ranging from discovery research to clinical trials to commercial applications. These products are critical raw materials and key components in commercially available drugs and medical devices. Aldevron specializes in GMP manufacturing and is known for inventing the GMP-SourceTM quality system. Company headquarters are in Fargo, North Dakota, with additional facilities in Madison, Wisconsin, and Freiburg, Germany. To learn more, visit www.aldevron.com.
About Oxford Genetics
Oxford Genetics is a leading synthetic biology company focused on developing novel technologies to overcome the challenges associated with the discovery, development and production of biologics, gene therapies, cell therapies and vaccines. Our proprietary genomic system enables the precise engineering of DNA, which in combination with our automated platforms, drive the rational design of complex biologics and their associated production processes with the overarching aim of increasing predictability, robustness and scalability, while lowering cost and human error.
View source version on businesswire.com: https://www.businesswire.com/news/home/20190123005452/en/
Contacts
Ellen Shafer
Senior Director of Marketing and Communications
ellen.shafer@aldevron.com
701.499.7386
Source: Aldevron







