Fresenius Kabi Introduces Glucagon Emergency Medicine Kit to Treat Life-Threatening Episodes of Low Blood Sugar

Glucagon Emergency Kit is a cost-effective alternative to currently marketed branded kit products for severe hypoglycemia
Co-pay assistance program available
LAKE ZURICH, Ill.--(BUSINESS WIRE)-- Fresenius Kabi announced today the availability in the United States of its Glucagon Emergency Kit, an FDA-approved and cost-effective alternative to treat severe hypoglycemic episodes in people with diabetes. The kit includes Glucagon for Injection 1 mg and a prefilled glass syringe with 1 mL of Sterile Water for Injection, USP.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20200205005106/en/
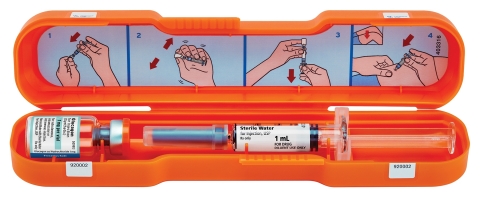
A Glucagon Emergency Kit is now available from Fresenius Kabi. The kit is designed to assist patients experiencing severe hypoglycemia. (Photo: Business Wire)
“For people with diabetes, a severe hypoglycemic episode can occur anywhere and at any time,” said John Ducker, president and CEO of Fresenius Kabi USA. “Our new Glucagon Emergency Kit allows clinicians flexibility and choice for treating patients experiencing severe hypoglycemia. This is another example of Fresenius Kabi’s commitment to providing cost-effective products that deliver life-saving care.”
The Glucagon Emergency Kit is designed to be convenient and easy to use. Patients can carry it with them, so it is available should they experience severe hypoglycemia. The bright orange case makes it easy for a patient or caregiver to find it and act quickly. To help make the Glucagon Emergency Kit more affordable, there is a co-pay assistance program for patients who qualify.
Fresenius Kabi is also making injection training kits available to health care providers to help educate patients. To learn more about the Glucagon Emergency Kit, please visit www.glucagonemergencykit.com.
Fresenius Kabi is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition. To learn more about Fresenius Kabi, including its expanding U.S. centers for pharmaceutical research, manufacturing and distribution, please visit www.fresenius-kabi.com/us.
About Glucagon Emergency Kit, USP
Glucagon for Injection is an antihypoglycemic agent indicated for the treatment of severe hypoglycemia in pediatric and adult patients with diabetes.
Important Safety Information
Glucagon for Injection is contraindicated in patients with pheochromocytoma, insulinoma, or a known hypersensitivity to glucagon or any of the excipients.
Catecholamine Release in Patients with Pheochromocytoma: Glucagon for Injection is contraindicated in patients with pheochromocytoma because Glucagon for Injection may stimulate the release of catecholamines from the tumor.
Hypoglycemia in Patients with Insulinoma: In patients with insulinoma, administration may produce an initial increase in blood glucose; however, Glucagon for Injection may stimulate exaggerated insulin release from an insulinoma and cause hypoglycemia. If a patient develops symptoms of hypoglycemia after a dose of Glucagon for Injection, give glucose orally or intravenously.
Hypersensitivity and Allergic Reactions: Allergic reactions have been reported and include generalized rash, and in some cases anaphylactic shock with breathing difficulties, and hypotension.
Lack of Efficacy in Patients with Decreased Hepatic Glycogen: Glucagon for Injection is effective in treating hypoglycemia only if sufficient hepatic glycogen is present. Patients in states of starvation, with adrenal insufficiency or chronic hypoglycemia may not have adequate levels of hepatic glycogen for Glucagon for Injection to be effective. Patients with these conditions should be treated with glucose.
Necrolytic Migratory Erythema (NME): a skin rash, has been reported postmarketing following continuous glucagon infusion and resolved with discontinuation of the glucagon. Should NME occur, consider whether the benefits of continuous glucagon infusion outweigh the risks.
Most common adverse reactions (>5% or greater incidence): Injection site swelling, injection site erythema, vomiting, nausea, decreased blood pressure, asthenia, headache, dizziness, pallor, diarrhea, and somnolence.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Beta-blockers: Patients taking beta-blockers may have a transient increase in pulse and blood pressure.
Indomethacin: In patients taking indomethacin Glucagon for Injection may lose its ability to raise glucose or may produce hypoglycemia.
Warfarin: Glucagon for Injection may increase the anticoagulant effect of warfarin.
This Important Safety Information does not include all the information needed to use Glucagon for Injection safely and effectively. Please see the full prescribing information for Glucagon for Injection. Full prescribing information is also available at www.fresenius-kabi.com/us.
About Fresenius Kabi
Fresenius Kabi (www.fresenius-kabi.com/us) is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition. The company’s products and services are used to help care for critically and chronically ill patients. The company’s U.S. headquarters is in Lake Zurich, Illinois. The company’s global headquarters is in Bad Homburg, Germany. To learn about U.S. career opportunities at Fresenius Kabi, visit us at https://www.fresenius-kabi.com/us/join-us.
View source version on businesswire.com: https://www.businesswire.com/news/home/20200205005106/en/
Source: Fresenius Kabi