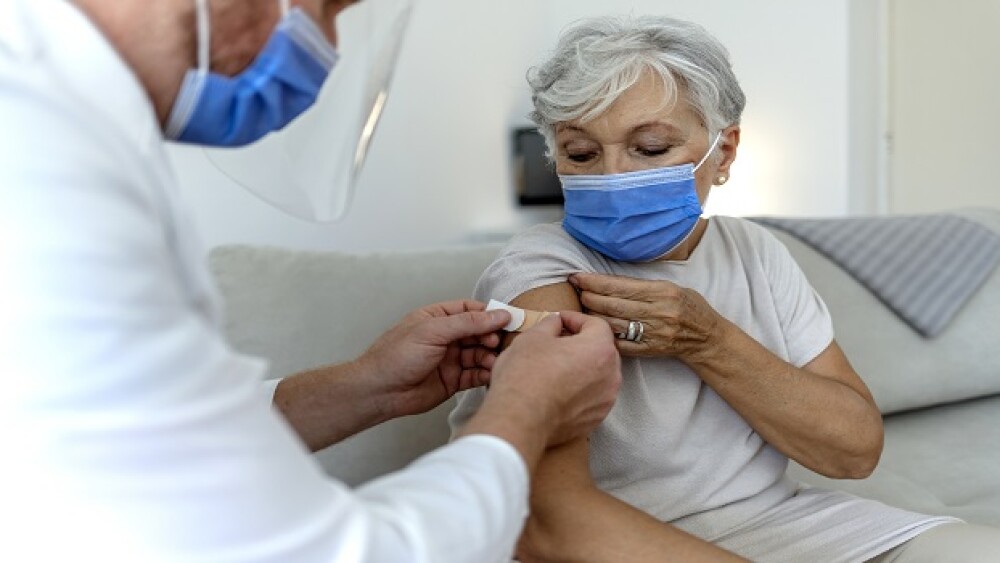
On Wednesday, the FDA authorized new Omicron-specific booster shots for Pfizer-BioNTech and Moderna’s COVID-19 vaccines despite a lack of human data.
The FDA authorized new Omicron-specific booster shots developed by Pfizer-BioNTech and Moderna Wednesday despite a lack of human data.
The new vaccine boosters are bivalent, containing messenger RNA (mRNA) that codes for both the spike protein of the original wildtype Wuhan strain and BA.1 or BA.4 and BA.5, which have identical spike proteins. There are no human data for the boosters, and some experts expressed concern.
In a meeting with the FDA’s vaccine advisory committee in June, the companies presented data demonstrating the boosters had side effects like those of the original vaccines, primarily site injection soreness and fatigue. They also stated the boosters elicited strong antibody responses to the original strain and Omicron BA.1, as well as to BA.4 and BA.5, although the response was lower in BA.4 and BA.5 than in BA.1.
Clinical trials for the BA.4/BA.5 vaccines will begin later in August.
Paul Offit, M.D., member of the FDA’s vaccine advisory committee and vaccine expert at Children’s Hospital of Philadelphia, told BioSpace that the data presented in June before the vaccines advisory committee was done the right way. While it increased immunogenicity by 1.5 to 1.75-fold in mice given vaccines targeting BA.1, it was “not likely to be a clinically meaningful benefit” in people, he said.
Offit also expressed concern over the lack of any human data for either of the bivalent vaccines in humans, which he said could be gathered in about a month. He asserted that the data does not represent a need for booster doses with the bivalent and Omicron-specific vaccines to prevent hospitalization in an otherwise healthy population, but might in the elderly or immunosuppressed.
Influenza vaccines, for example, are reformulated each year and don’t undergo new clinical trials unless the vaccine makers significantly modify the way they manufacture the vaccines. However, Offit said, “We’ve known exactly what the flu shots do since the 1940s. But the mRNA vaccines have only been around for less than two years and we have no data in humans on the bivalent vaccines. We can’t assume. The history of medical innovation would suggest we should be cautious.”
Still, not all experts are concerned. Leif Erik Sander, M.D., an infectious disease expert at the Charité University Hospital in Berlin, told Science the changes to the mRNA are minor and that an updated vaccine created as quickly as possible is “an ethical issue,” adding, “We need to allow people to protect themselves from a virus that we can’t fully control.”
The decisions will now go to the CDC and its Advisory Committee on Immunization Practices for a recommendation on their use. If they agree and CDC Director Rochelle Walensky, M.D., concurs, the booster shots could become available after Labor Day.
“A great deal of care has been taken by the FDA to ensure that these updated boosters meet our rigorous safety, effective and manufacturing quality standards for emergency use authorization,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research (CBER), in a media call on Wednesday.
The FDA recommends the boosters for those who received the original two-dose regimen of either vaccine and those who received the initial two doses with one or two previous boosters. The new vaccine boosters should be received at least two months after the last shots.
Marks indicated the two-month period was recommended to ensure the body would build a sufficient immune response. It also would decrease the risk of myocarditis, a rare heart inflammatory condition that has been associated with both vaccines, but also with COVID-19, he said.
The U.S. government has already entered an agreement to acquire 105 million doses of the Pfizer-BioNTech booster and 66 million of the Moderna shots.
The original Omicron BA.1 variant is no longer circulating. Beginning in spring 2022, subvariants of Omicron, BA.4 and BA.5, outpaced their parent variant. In June, the FDA asked vaccine manufacturers to develop boosters to specifically target BA.4 and BA.5.
For the boosters containing BA.4 and BA.5 mRNA, the companies submitted preclinical animal data, which have not been released to the public. At the June meeting, Pfizer presented early findings in eight mice given BA.4/BA.5 shots as a third dose. Compared to the laboratory animals receiving the original vaccine as a booster, the BA.4/BA.5 booster recipients demonstrated an increased response to all Omicron variants: BA.1, BA.2, BA.2.12.1, BA.4 and BA.5.