AIM ImmunoTech Inc. (NYSE American: AIM) today released detailed safety data from a Phase 1 clinical study which supports the company’s belief that its drug Ampligen has significant potential as an intranasal therapeutic for COVID-19.
OCALA, Fla., Oct. 06, 2021 (GLOBE NEWSWIRE) -- AIM ImmunoTech Inc. (NYSE American: AIM) today released detailed safety data from a Phase 1 clinical study which supports the company’s belief that its drug Ampligen has significant potential as an intranasal therapeutic for COVID-19.
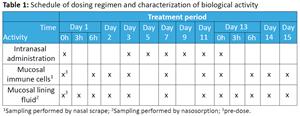
AIM previously announced that the study to assess the safety, tolerability and biological activity of Ampligen as a potential intranasal therapy was completed, and that a Safety Report found the drug to be generally well tolerated and reported no Severe Adverse Events. More details are now being published on both the AIM website and on the website for the Centre for Human Drug Research (CHDR). A total of 40 healthy subjects received either Ampligen or a placebo in the trial, with the Ampligen given at four escalating dosages across four cohorts, to a maximum level of 1,250 micrograms. The poster was recently presented at the FIGON Conference in the Netherlands. Dosing was administered every other day (Table 1) for 7 doses.
Table 1 is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/32c54008-065e-4609-9dde-ced2b0014b4c
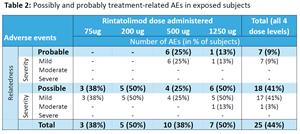
The safety results (Table 2) shows that no severe adverse events were reported and found the drug to be generally well tolerated.
Table 2 is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/0abc4748-3728-4e69-aa96-04e98548aee9
The biological samples are being analyzed for non-safety-related immunological activity, with results expected in Q4 2021.
Based on these positive safety results, AIM has moved forward in its efforts to develop Ampligen as a potential intranasal treatment for COVID-19, including:
- A Phase 2a Human Challenge Trial (HCT) to test Ampligen as a potential intranasal prophylactic using a human rhinovirus (HRV-16, a common cold virus) and influenza A virus (H3N2), which could help establish Ampligen as a potential prophylaxis against future viral variants and future novel respiratory viruses for which there are no current therapies, as well as mutations of known viruses such as SARS-CoV-2, which causes COVID-19
- A Pre-Investigational New Drug application (Pre-IND) to the U.S. Food and Drug Administration (FDA) for two separate Phase 2 clinical studies to study the potential of Ampligen as both an intranasal and an infusion treatment for early-onset COVID-19
- The filing of a provisional patent application for Ampligen as a potential early-onset intranasal therapy designed to enhance and expand infection-induced immunity, epitope spreading, cross-reactivity and cross-protection in patients exposed to a wide range of RNA respiratory viruses
About AIM ImmunoTech Inc.
AIM ImmunoTech Inc. is an immuno-pharma company focused on the research and development of therapeutics to treat multiple types of cancers, immune disorders, and viral diseases, including COVID-19, the disease caused by the SARS-CoV-2 virus.
Cautionary Statement
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 (the “PSLRA”). Words such as “may,” “will,” “expect,” “plan,” “anticipate” and similar expressions (as well as other words or expressions referencing future events or circumstances) are intended to identify forward-looking statements. Many of these forward-looking statements involve a number of risks and uncertainties. Among other things, for those statements, the Company claims the protection of safe harbor for forward-looking statements contained in the PSLRA. No assurances can be given as to whether any studies will be successful or yield favorable data. Studies and trials are subject to many factors including lack of regulatory approval(s), lack of study drug, or a change in priorities at the institutions sponsoring other trials. Significant additional testing and trials will be required to determine whether Ampligen will be effective in the treatment of respiratory viruses, including SARS-CoV-2, as an intranasal therapy or otherwise, and no assurance can be given that this will be the case. There is the potential for delays in clinical trial enrollment and reporting because of the COVID-19 medical emergency. We do not undertake to update any of these forward-looking statements to reflect events or circumstances that occur after the date hereof.
Contacts:
Crescendo Communications, LLC
Phone: 212-671-1021
Email: aim@crescendo-ir.com
AIM ImmunoTech Inc
Phone: 800-778-4042
Email: IR@aimimmuno.com