Nanjing Leads Biolabs Co., Ltd. announced that LBL-024, an anti-PD-L1/4-1BB bispecific antibody independently developed by Leads Biolabs with global intellectual property rights has received approval to conduct the single-arm pivotal study for registration and market authorization from the Center for Drug Evaluation of the National Medical Products Administration.
NANJING, China, April 30, 2024 /PRNewswire/ -- Nanjing Leads Biolabs Co., Ltd. (hereinafter referred to as "Leads Biolabs") announced that LBL-024, an anti-PD-L1/4-1BB bispecific antibody independently developed by Leads Biolabs with global intellectual property rights has received approval to conduct the single-arm pivotal study for registration and market authorization from the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA). Currently, there are no similar products approved for marketing domestically or internationally.
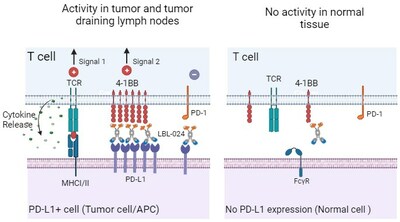
LBL-024 is a bispecific antibody composed of anti-programmed death ligand-1 (PD-L1) and anti-4-1BB (CD137) antibodies. It binds to PD-L1 with high affinity, blocking the PD-L1/PD-1 immunosuppressive pathway while conditionally activating the 4-1BB costimulatory pathway in the tumor microenvironment. This activation of T cells exerts a powerful immune response, resulting in a stronger antitumor effect than anti-PD-1/PD-L1 monoclonal antibodies alone.
LBL-024 received IND approvals from both FDA and NMPA on July 30, 2021 and September 9, 2021 respectively to conduct phase Ⅰ/Ⅱ clinical research, and has achieved outstanding results. The clinical study results of LBL-024 monotherapy in patients with advanced malignant tumors demonstrated good safety profile and encouraging efficacy signals in the advanced solid tumors.
The approved Phase IIb pivotal clinical study is led by Professor Shen Lin from Peking University Cancer Hospital, and detailed clinical data will be disclosed during the ASCO Annual Meeting on May 31st to June 4th, 2024. LBL-024 has First-in-Class potential and is expected to offer an effective treatment option to patients with advanced solid tumors.
Dr. Charles Cai, Chief Medical Officer of Leads Biolabs, said " The approval of this pivotal clinical study is an encouraging news. Based on the current treatment status and the available safety and efficacy data, LBL-024 meets the requirements of the drug registration management measures for 'innovative drugs used to prevent and treat diseases that seriously endanger life or severely affect the quality of life, and for which there are no effective prevention or treatment methods, or there is sufficient evidence to demonstrate significant clinical advantages compared to existing treatment methods.' The approval of the single-arm pivotal clinical study by CDE will help accelerate the marketing process of LBL-024 and bring more effective treatment options to patients as early as possible."
Dr. Xiaoqiang Kang, founder, chairman and CEO of Leads Biolabs, said " We are delighted to see the positive progress of LBL-024, which has the potential to become an effective immunotherapy and prove 4-1-BB to be druggable immune checkpoint target approved for marketing following PD-1/PD-L1, CTLA-4, and LAG-3. This milestone achievement also reflects our corporate philosophy of focus on innovation and our determination to discover and advance First-in-Class products. We have always been guided by clinical needs in our differentiated approach to innovation, deploying novel targets while combining some targets based on a deep understanding of their biological mechanisms and disease mechanisms to achieve a 1+1>2 effect. The vision of Leads Biolabs is to become a company that can truly develop innovative drugs, delivering safe, effective, and accessible new therapies to patients worldwide. To this end, we will continue to make full efforts to support the subsequent clinical research of LBL-024, and look forward to delivering the benefit earlier to patients around the world. "
About Leads Biolabs
Nanjing Leads Biolabs Co., Ltd. is a clinical-stage biotechnology company founded in Nanjing by a team of senior U.S.-trained antibody drug developers. Since 2014, Leads Biolabs has been dedicated to the discovery and development of novel antibody drugs with independent intellectual property rights for the treatment of oncology and other major diseases of high unmet medical needs, particularly the challenges in cancer immunotherapy. Our extensive R&D pipeline consist of more than twenty novel tumor immunotherapy, autoimmunity and ADC molecules based on monoclonal and bispecific antibody technology platforms. Leads Biolabs is committed to providing safe, effective, accessible and affordable new drugs to address the unmet needs of patients around the world.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/a-potential-first-in-class-drug-cde-approved-single-arm-pivotal-clinical-study-of-lbl-024-an-anti-pd-l14-1bb-bispecific-antibody-developed-by-leads-biolabs-302131599.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/a-potential-first-in-class-drug-cde-approved-single-arm-pivotal-clinical-study-of-lbl-024-an-anti-pd-l14-1bb-bispecific-antibody-developed-by-leads-biolabs-302131599.html
SOURCE LEADS BIOLABS