zEEG is a Comfortable, No-Residue, Wireless EEG Headset Approved by FDA for Clinical Use
zEEG is a Comfortable, No-Residue, Wireless EEG Headset Approved by FDA for Clinical Use
SANTA CLARA, Calif., March 10, 2020 /PRNewswire/ -- Zeto, Inc., a privately held medical technology company that is transforming the Electroencephalography (EEG) experience for healthcare, today announced that it has raised $7.3 million in Series A financing led by Seraph Group and joined by Aphelion Capital, SV Tech Ventures and Shangbay Capital. Zeto raised $4 million previously in dilutive and non-dilutive funding. The new capital will be used to accelerate commercialization of its lead product, the zEEG headset and software platform, and develop new products to expand its portfolio. zEEG is the first true dry electrode EEG headset to be approved by the U.S. Food and Drug Administration (FDA) for clinical use.
"Since our commercial launch a few months ago, we added several customers who love the product and are utilizing it at high rates. We are glad to validate the strong product-market fit for zEEG. Inspired by the demand, we are excited to expand our commercial team and production capacity to serve our growing customer base," said Aswin Gunasekar, MS, MBA, Chief Executive Officer and Founder of Zeto. "Our goal is to provide the most seamless and intelligent EEG experience for any clinical need. Current products are far behind what users need and Zeto is building a suite of convenient and intuitive products to eliminate the most time consuming and labor-intensive activities involved in traditional EEG. As a subsequent step, we plan to utilize artificial intelligence to not only supplement the reading physician with analytics and insight but also expand the diagnostic utility of EEG worldwide in areas of medicine where it has been underutilized."
Zeto's customer, Wave Neuroscience, Inc., said, "Our extensive intellectual property portfolio and business operations are built on award winning science and medical innovation. For us to achieve the high level of results we are committed to, we rely on partners that never rest in their quest to achieve the highest standards. Accessing an FDA compliant EEG device that performs at an uncompromising standard, allows for increased throughput and provides a user experience that rivals the latest technology assets, was very attractive to us. Zeto transformed the way we think about EEG capture by advancing technology further in the past two years than the industry had in the prior three or four decades. Zeto provides us the confidence, convenience and data quality that we rely on, day in and day out."
"We believe a new era is around the corner when researchers can accelerate our understanding of the brain with this breakthrough device and software platform. It not only makes commercial sense, but also can serve as the infrastructure and library for neurological knowledge that leads to better diagnosis and treatment of brain disorders," said Tuff Yen, CEO of Seraph Group, the lead investor.
"We are passionate about brainwaves, consider it a privilege to serve our customers and are committed to bringing great advancements for neurological care," adds Aswin Gunasekar.
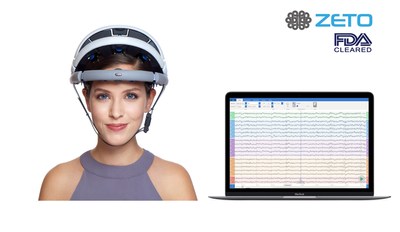
About zEEG
zEEG is a commercially available wireless EEG headset backed by a cloud platform that offers instant upload, tools for analysis and remote interpretation by neurologists. The zero-prep, easy to wear zEEG headset has 20 dry electrodes that can be positioned as per the 10-20 EEG system. zEEG was approved for clinical use by the U.S. Food and Drug Administration (FDA) in April 2018.
About Zeto, Inc.
Zeto, Inc., is a privately held medical technology company located in Santa Clara, CA focused on transforming the way electroencephalography (EEG) is performed at hospitals and clinics. Zeto's revolutionary platform brings the traditional EEG procedure to the 21st century. The company plans to leverage its hardware and software technology to improve noninvasive monitoring of brain's electrical activity and achieve better outcomes for neurological conditions such as epilepsy, sleep disorders, autism, stroke and concussion.
Media Contact:
Gina Nugent, Nugent Communications
gina@nugentcommunications.com
617-460-3579
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/zeto-raises-series-a-funding-to-accelerate-commercialization-efforts-of-its-easy-use-electroencephalography-eeg-headset-and-software-platform-301020172.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/zeto-raises-series-a-funding-to-accelerate-commercialization-efforts-of-its-easy-use-electroencephalography-eeg-headset-and-software-platform-301020172.html
SOURCE Zeto, Inc.