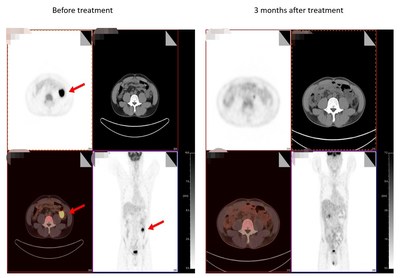
On September 25, 2020, BIORAY LABORATORIES Inc. announced for the first time that clinical trials of non-viral PD1 specific targeted CAR T therapy in relapsed/refractory B-cell non-Hodgkin lymphoma, undertaken by the company in cooperation with East China Normal University and the First Affiliated Hospital, School of Medicine, Zhejiang University, has achieved significant breakthroughs.
SHANGHAI, Sept. 28, 2020 /PRNewswire/ -- On September 25, 2020, BIORAY LABORATORIES Inc. ("Bioraylab"), a company specialized in gene therapy and cell drug R&D, announced for the first time that clinical trials of non-viral PD1 specific targeted CAR T therapy in relapsed/refractory B-cell non-Hodgkin lymphoma, undertaken by the company in cooperation with East China Normal University and the First Affiliated Hospital, School of Medicine, Zhejiang University, has achieved significant breakthroughs. This is the world's first application of gene editing technology to realize PD1-knockin CAR T treatment, and is also the world's first clinical trial treating lymphoma with non-viral PD1 specific targeted CAR T cells.
The latest research results, finished by East China Normal University, the First Affiliated Hospital, School of Medicine, Zhejiang University and Bioraylab, were announced on the preprint platform medRxiv on September 23, 2020.
PD1 specific targeted CD19-CART is Bioraylab's Quikin CART platform technology using its proprietary IP, precisely inserting CAR cassette into PD1 locus without using virus, thus generating the CAR T product in just one step. The product combines PD1 immune checkpoint inhibition with CART anti-tumor activity, generating effects of both anti-PD1 immunotherapy and CAR T therapy. Several ongoing clinical trials demonstrate the outstanding safety and effectiveness of this CAR T product.
Two patients showed complete remission following a three-month treatment
The clinical trial program enrolled 15 patients, among which, four patients who were able to be evaluated showed partial remission (PR) after receiving the one-month treatment, while two patients showed complete remission (CR) after three months of treatment.
There were no CAR T cell related high-grade (≥3) adverse events during the entire treatment, including cytokine release syndrome (CRS) and neurologic toxicity. After infusion, the CAR T cells were well sustained in vivo.
The Quikin CART technology produces CAR T cells without using virus, greatly reducing high costs of producing CAR T products, while avoiding the cancer risk from random insertion. The regulation of T cell endogenous genes and the constant expression of CAR are realized in just one step. Compared with other CAR T technologies, Quikin CART has a variety of advantages, including a simpler process, fewer production links, shorter time preparation and higher product uniformity.
Liu Mingyao, chief scientist at Bioraylab and professor at East China Normal University, said, "Without using virus, the Quikin CART technology can realize the precise integration of CAR cassette into genome as well as regulation of T cell endogenous genes in just one step, delivering many unparalleled advantages compared with existing CAR T technologies. The technology offers a strong platform for the development of more diverse CAR T products in the future."
Huang He, the project investigator of this clinical trial and chairman of the First Affiliated Hospital, School of Medicine, Zhejiang University, commented, "The current results showed that the non-viral PD1-knockin CAR T cells produced by using the Quikin CART technology have great potential to treat patients. We are very delighted to see patients rapidly show CR after treatment, and are looking forward to safer and more durable long-term effects on relapsed/refractory patients through this novel CAR T technology."
In addition to the ongoing clinical trials of non-viral PD1 specific targeted CD19-CART, Bioraylab is conducting research on other non-viral specific targeted CART products for treating solid tumors, with the aim of achieving more breakthroughs in CAR T therapy.
About Bioraylab
Founded in Shanghai in 2013, Bioray Laboratories Inc. ("Bioraylab") is already well positioned to become the world's leading gene cell pharmaceutical manufacturer, by way of innovation with gene editing and the development of breakthrough therapies that benefit all mankind. Bioraylab owns 108 patents, conducted IIT trials for five projects in eight of the world's leading hospitals, three of which having entered the IND application stage. Bioraylab has, over the past five years, published 12 high-level academic papers in internationally renowned journals, including Nature Biotechnology and Nature Medicine. The firm has built three technology platforms for gene editing, cell therapy and gene therapy, and has a 6,000-square-meter GMP pilot plant and an operating team comprising nearly 100 people, placing the firm in the position of being able to guarantee the rapid transformation and application of innovative research results.
View original content to download multimedia:http://www.prnewswire.com/news-releases/worlds-first-clinical-trial-of-non-viral-pd1-specific-targeted-car-t-therapy-achieves-great-breakthroughs-301138855.html
SOURCE Bioray Laboratories Inc.