TUCSON, Ariz., June 4, 2014 /PRNewswire/ -- Ventana Medical Systems, Inc. (Ventana), a member of the Roche Group and MedImmune, the global biologics research and development arm of AstraZeneca, today announced they are jointly developing a PD-L1 (SP263) immunohistochemistry assay to enroll patients in clinical trials for MedImmune's MEDI4736 anti-PD-L1 therapy for non-small cell lung carcinoma. This includes the recently commenced MEDI4736 ATLANTIC trial that will enroll only patients who express PD-L1 as determined by the VENTANA assay.
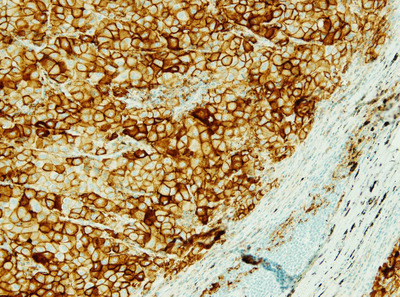
MEDI4736 is an investigational, engineered, human monoclonal antibody directed against programmed cell death ligand 1 (PD-L1). Signals from PD-L1 help tumors avoid detection by the immune system. It is believed that by targeting PD-L1, MEDI4736 may block this ligand from sending out signals to T-cells to 'ignore' tumor cells, thereby countering cancer's immune-evading tactics.
The PD-L1 Investigational Use Only (IUO) assay in development that is supporting MEDI4736 clinical trials- is based on a SP263 (Spring Bioscience) rabbit monoclonal antibody.
"Cancer immunotherapy is a promising area that may provide long lasting benefit not achieved with other treatment approaches. This collaboration is another strong example of the importance of companion diagnostics and their role in supporting the use of novel immunotherapies to target human malignancies," says Doug Ward, VP and Lifecycle Leader for the Ventana Companion Diagnostics business unit. "We're proud to provide high quality reagents like SP263 to support MedImmune's immunotherapy development efforts to advance the standard of care for cancer patients."
The PD-L1 (SP263) assay has been validated for use on the VENTANA BenchMark series of advanced staining instruments deployed globally. Testing for MedImmune's trials is being performed at the Ventana companion diagnostics (CDx) CAP/CLIA laboratory in Tucson, AZ, and select testing sites.
"Personalized healthcare is an important part of our development strategy, so we are pleased to collaborate with Ventana on this companion diagnostic to help further advance our MEDI4736 development program," said Dr. Edward Bradley, Senior Vice President, R&D and Oncology iMED Head, MedImmune.
Since 2002 Ventana has worked with more than 45 biopharmaceutical partners and is currently engaged in more than 180 collaborative projects to develop and commercialize companion diagnostics globally. The company has a global install base of over 10,000 automated platforms that run advanced cancer diagnostic tests to benefit patients.
About the Ventana CAP/CLIA Lab
The Ventana CAP/CLIA lab is a full-service histopathology lab that employs automated staining platforms and validated assays for IHC and ISH staining of retrospective and prospective clinical samples. The laboratory's goal is to support hypothesis testing evaluated in non-pivotal and pivotal clinical trials. Customers for these services include external pharmaceutical companies that are interested in oncology biomarker evaluation and companion diagnostic development using tissue-based IHC and ISH assays.
About Ventana Medical Systems, Inc.
Ventana Medical Systems, Inc. ("VMSI") (SIX: RO, ROG; OTCQX: RHHBY), a member of the Roche Group, innovates and manufactures instruments and reagents that automate tissue processing and slide staining for cancer diagnostics. VENTANA products are used in clinical histology and drug development research laboratories worldwide. The company's intuitive, integrated staining, workflow management platforms, and digital pathology solutions optimize laboratory efficiencies to help reduce errors, support diagnosis and enable informed treatment decisions by anatomic pathology professionals. Together with Roche, VMSI is driving Personalized Healthcare through accelerated drug discovery and the development of "companion diagnostics" to identify the patients most likely to respond favorably to specific therapies. VENTANA, the VENTANA logo and BenchMark are trademarks of Roche.
About Spring Bioscience
Spring Bioscience (Spring) was founded in 2001 by a team of scientists and achieved early success with their line of SP Clone rabbit monoclonal antibodies for clinical immunohistochemistry. Ventana Medical Systems, Inc. acquired the company in 2007 for its antibody development capabilities. Today Spring serves an important role as an antibody center of excellence in support of Roche's mission to deliver companion diagnostics. This is accomplished through the development of antibodies destined for clinical assays through Ventana and other Roche affiliates and through its collective medical and market intelligence serving the cancer research community.
About MedImmune
MedImmune is the global biologics research and development arm of AstraZeneca, a global, innovation-driven biopharmaceutical business that focuses on the discovery, development and commercialization of small molecule and biologic prescription medicines. MedImmune is pioneering innovative research and exploring novel pathways across key therapeutic areas, including respiratory, inflammation and autoimmunity; cardiovascular and metabolic disease; oncology; neuroscience; and infection and vaccines. The MedImmune headquarters is located in Gaithersburg, Md., one of AstraZeneca's three global R&D centers. For more information, please visit www.medimmune.com.
VMSI Media Relations
Jacqueline Bucher
Senior Director, Corporate Communications
Phone: 520-877-7288
e-mail: Jacquie Bucher
MedImmune Media Relations
Tracy Rossin
Director, External Communications
Phone: 301-412-8236
e-mail: rossint@medimmune.com
Photo - http://photos.prnewswire.com/prnh/20140602/93465
SOURCE Ventana Medical Systems, Inc.
Help employers find you! Check out all the jobs and post your resume.




