ROCKVILLE, Md., Oct. 8, 2015 /PRNewswire/ -- Synthetic Biologics, Inc. (NYSE MKT: SYN), a clinical-stage company focused on developing therapeutics to protect the microbiome while targeting pathogen-specific diseases, reported the initiation of the second Phase 2 clinical trial of its proprietary SYN-010 for the treatment of irritable bowel syndrome with constipation (IBS-C). This multi-center, open-label study will evaluate the sustainability of the effect of one dose strength of SYN-010 on breath methane production in breath methane-positive patients with IBS-C. The study will also evaluate key clinical outcomes, including frequency of complete spontaneous bowel movements (CSBM), abdominal pain and bloating. The presence of breath methane has been associated with pain, bloating and constipation in IBS-C patients, and published reports have associated higher intestinal methane production with increased constipation severity in IBS-C patients.
IBS affects an estimated 10 to 15 percent of the population, or as many as 45 million people in North Americai. The illness affects both men and women; however, two-thirds of diagnosed sufferers are women. Current U.S. Food and Drug Administration (FDA)-approved therapies for the treatment of IBS-C and other treatments including prescription and over-the-counter laxatives, provide patients with temporary symptomatic relief, but do not treat the underlying cause of pain, bloating and constipation associated with IBS-C.
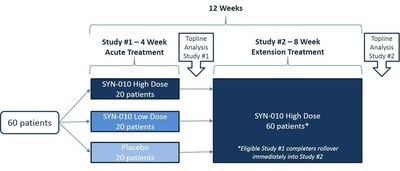
"Synthetic Biologics' IBS-C program is moving through the clinic as planned. Patient enrollment in the first Phase 2 clinical trial of SYN-010 is complete and all patients have received their first dose according to the randomization protocol," stated Jeffrey Riley, Chief Executive Officer of Synthetic Biologics. "As the patients complete the first Phase 2 clinical trial, they are eligible to immediately rollover into the second Phase 2 clinical trial of SYN-010 that will evaluate the ability of SYN-010 to sustain the reduction in breath methane levels, and the frequency of CSBM, abdominal pain and bloating. Current treatments are focused on relieving symptoms, whereas SYN-010 has therapeutic potential to reduce the production of methane in the gut, treating a major underlying cause of IBS-C."
Mr. Riley concluded, "At this time, more than half of the total participants have completed the first Phase 2 clinical trial and rolled over into the second Phase 2 clinical trial of SYN-010. We continue to anticipate reporting topline results from the first Phase 2 clinical trial of SYN-010 during the fourth quarter of 2015, and reporting topline results from the second Phase 2 clinical trial during the first half of 2016."
SYN-010 is a proprietary, modified-release formulation of the classic statin, lovastatin, that is intended to reduce methane production by certain microorganisms (M. smithii) in the gut while minimizing disruption to the microbiome. Methane produced by M. smithii is perceived as the underlying cause of pain, bloating, and constipation associated with IBS-C. SYN-010 is intended to act primarily in the intestinal lumen while avoiding systemic absorption, thereby targeting the major cause of IBS-C, not just the symptoms. To access the SYN-010 mechanism of action video on Synthetic Biologics' website, please click here.
Second SYN-010 Phase 2 Clinical Trial Design
The second Phase 2 open-label clinical trial is being conducted at multiple centers in the United States. The primary objective of this study is to evaluate the sustainability of the effect of one dose strength of SYN-010 on breath methane production in breath methane-positive patients with IBS-C. Secondary objectives include evaluating the reduction in abdominal pain and bloating, and the increase in CSBM. Approximately 60 patients, who complete the first Phase 2 clinical trial of SYN-010, are eligible to immediately rollover into the second Phase 2 clinical trial of SYN-010. Patients are scheduled to receive a single oral dose of SYN-010 each day for approximately two months.
About Synthetic Biologics, Inc.
Synthetic Biologics, Inc. (NYSE MKT: SYN) is a microbiome-focused, clinical-stage company developing therapeutics to protect the microbiome while targeting pathogen-specific diseases. The Company's lead candidates in Phase 2 development include SYN-004 which is designed to protect the gut microbiome from the effects of certain commonly used intravenous (IV) antibiotics for the prevention of C. difficile infection and antibiotic-associated diarrhea (AAD), and SYN-010 which is intended to reduce the impact of methane producing organisms in the gut microbiome to treat the underlying cause of irritable bowel syndrome with constipation (IBS-C). In addition, the Company is developing a Phase 2 oral estriol drug for the treatment of relapsing-remitting multiple sclerosis (MS) and cognitive dysfunction in MS, and in collaboration with Intrexon Corporation (NYSE: XON), a monoclonal antibody combination for the treatment of Pertussis and biotherapeutics for the treatment of phenylketonuria (PKU). For more information, please visit Synthetic Biologics' website at www.syntheticbiologics.com.
This release includes forward-looking statements on Synthetic Biologics' current expectations and projections about future events. In some cases forward-looking statements can be identified by terminology such as "may," "should," "potential," "continue," "expects," "anticipates," "intends," "plans," "believes," "estimates," and similar expressions. These statements are based upon current beliefs, expectations and assumptions and are subject to a number of risks and uncertainties, many of which are difficult to predict and include statements regarding the potential of SYN-010 to reduce methane production for the treatment of IBS-C and address an unmet need, the ability of SYN-004 to protect the microbiome, the size of the market,the anticipated timing of the reporting of topline data from the first Phase 2 clinical trial of SYN-010 during the fourth quarter of 2015, and reporting topline results from the second Phase 2 clinical trial. The forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially from those set forth or implied by any forward-looking statements. Important factors that could cause actual results to differ materially from those reflected in Synthetic Biologics' forward-looking statements include, among others, a failure to receive the necessary regulatory approvals for commercialization of Synthetic Biologics' therapeutics, a failure of Synthetic Biologics' clinical trials, and those conducted by investigators, to be commenced or completed on time or to achieve desired results, a failure of Synthetic Biologics' clinical trials to receive anticipated funding, a failure of Synthetic Biologics' products for the prevention and treatment of diseases to be successfully developed or commercialized, Synthetic Biologics' inability to maintain its licensing agreements, or a failure by Synthetic Biologics or its strategic partners to successfully commercialize products and other factors described in Synthetic Biologics' report on Form 10-K for the year ended December 31, 2014 and any other filings with the SEC. The information in this release is provided only as of the date of this release, and Synthetic Biologics undertakes no obligation to update any forward-looking statements contained in this release on account of new information, future events, or otherwise, except as required by law.
i The International Foundation for Functional Gastrointestinal Disorders. http://www.aboutibs.org/.
Accessed June 19, 2015.

Photo - http://photos.prnewswire.com/prnh/20151007/275143
Video - http://youtu.be/-lxzseth6Z8
Logo - http://photos.prnewswire.com/prnh/20130522/MM19465LOGO
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/synthetic-biologics-initiates-second-syn-010-phase-2-clinical-trial-intended-to-treat-irritable-bowel-syndrome-with-constipation-ibs-c-300156246.html
SOURCE Synthetic Biologics, Inc.
 Help employers find you! Check out all the jobs and post your resume.
Help employers find you! Check out all the jobs and post your resume.




