SUNNYVALE, CA--(Marketwired - October 02, 2014) - Spiracur, the developer of the only mechanically powered advanced wound care solution that delivers the proven efficacy of Negative Pressure Wound Therapy (NPWT), today announced that it has entered into a three-year strategic agreement with HPSI Purchasing Services, whereby HPSI will make available to its members the SNaP® Wound Care System.
"The SNaP System and accompanying products from Spiracur will provide our more than 9,300 members in the senior living market with one of the industry's leading wound care solutions for the treatment and management of chronic and acute wounds, while complementing our entire product portfolio offering," said W. Greg Perron, senior vice president, contract administration for HPSI.
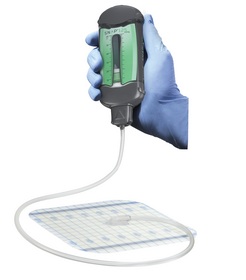
Spiracur's SNaP System and its suite of accessories combine the benefits of advanced wound care with non-powered NPWT, providing patients with a sophisticated, non-invasive and disposable NPWT system designed to promote wound healing. The small device weighs 2.2 ounces and does not require batteries or electricity. In addition, the SNaP System is silent and may be easily worn under clothing.
"Our partnership with HPSI affords us an entry into a new care setting, which is a critical part of our growth strategy in the next several years," said Chris Fashek, president and chief executive officer for Spiracur. "This agreement helps to strengthen our position in the wound care marketplace, and it expands our presence amongst the rapidly growing senior care market. Spiracur is dedicated to helping group purchasing organizations provide their members with effective solutions to enhance patient care, realize efficiencies and reduce expenses through clinically proven wound care products, and we are excited to have the SNaP System available for the first time in long-term care and skilled nursing facilities."
HPSI is a fully integrated group purchasing organization based in Irvine, Calif., with more than 9,300 nursing homes, assisted living facilities, residential care homes, skilled care facilities, independent living communities, retirement communities, and other senior care providers in its health care membership.
Spiracur has more than 88 million commercial covered lives, nearly 87 million Government covered lives, and the SNaP System is reimbursed in all 50 states.
About Spiracur Inc.
Spiracur Inc., headquartered in Sunnyvale, Calif., is a privately held medical device company focused on the development of innovative wound healing technologies. Spiracur was founded out of the Stanford University Biodesign Innovation Program in 2007. Its first product, the SNaP Wound Care System for the treatment of chronic and acute wounds, is the result of patient and clinician feedback that current negative pressure wound therapies were too cumbersome. The company's ciSNaP® Closed Incision System with proprietary controlled tension relief is designed to improve healing of high-risk surgical incisions. Both devices have received 510(k) clearance from the U.S. Food & Drug Administration (FDA) and have received CE Mark approval. Spiracur's proprietary mechanically powered technology does not require batteries or electricity to operate. For more information, please visit http://spiracur.com.
Spiracur, Spiracur logo and SNaP are registered trademarks of Spiracur Inc.
Media Contact:
Amy Cook
925.552.7893
Email Contact
Investor Contact:
Steve Van Dick
408.701.5300
Email Contact
Help employers find you! Check out all the jobs and post your resume.