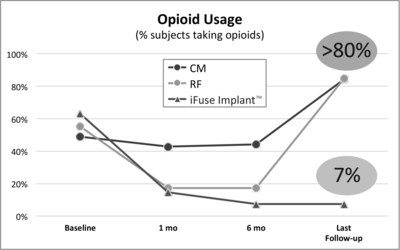
SAN JOSE, Calif., May 1, 2017 /PRNewswire/ -- SI-BONE, Inc., an innovative medical device company that pioneered the use of the iFuse Implant System® ("iFuse"), a triangular shaped minimally invasive surgical (MIS) device indicated for fusion for certain disorders of the sacroiliac (SI) joint, announced results from two recently published comparative studies showed patients treated with the iFuse ImplantTM were significantly less likely to be taking opioid medications than patients treated with non-surgical care. The most recently published paper titled Minimally Invasive Sacroiliac Joint Fusion, Radiofrequency Denervation and Conservative Management for Sacroiliac Joint Pain: Six Year Comparative Study,1 published in the journal Neurosurgery, showed that patients treated with iFuse were 11X less likely to be taking opioids at last follow-up than those who were denied coverage for iFuse and treated with either conservative care or radiofrequency ablation (>80% vs, 7%) (Figure 1, below).
"Given the catastrophic opioid addiction epidemic we are currently dealing with in this country today, any procedure, device or technology that demonstrates the ability to significantly reduce opioid use should be made available to anyone who can benefit," said Frank Guinta, former New Hampshire congressman and co-founder of the Congressional Bipartisan Committee on opioid and heroin addiction. "Given the overwhelming amount of high quality level 1 clinical evidence associated with the iFuse Implant, it seems prudent and obvious to me that anyone properly diagnosed as an appropriate surgical candidate should have access to the procedure."
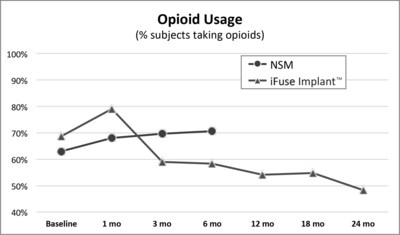
A second publication of two-year results from INSITE,2 a randomized controlled trial of MIS SI joint fusion with the iFuse Implant compared to non-surgical management, showed a 30% decrease from baseline in the number of subjects taking opioids at two years compared to patients treated non-surgically (Figure 2, below).
"It's rather remarkable that in both studies the number of patients taking opioids in the iFuse Implant group was significantly lower than the number of patients taking opioids in the non-surgical care group in spite of a lack of a structured program focused on opioid use dependence," said Daniel Cher, MD, Vice President of Clinical Affairs at SI-BONE. "It's clear from the evidence in these two studies that treatment with the iFuse Implant was associated with a reduction in opioid use in patients with chronic SI joint pain who were taking opioids and who no longer responded to non-surgical care."
About SI-BONE, Inc.
SI-BONE, Inc. (San Jose, California) is a leading innovative medical device company dedicated to the development, manufacture and commercialization of minimally invasive surgical devices for the treatment of patients with low back symptoms related to certain sacroiliac (SI) joint disorders. SI-BONE, Inc. first received 510(k) clearance to market its iFuse Implant System ("iFuse") from the Food and Drug Administration (FDA) in November 2008. The CE mark for European commercialization was obtained in November 2010.
The iFuse Implant System provides a minimally invasive surgical solution to fuse the SI joint using patented triangular titanium implants that create an interference fit within the ilium and sacrum. The triangular implant shape, combined with the press fit insertion, is designed to provide immediate fixation by minimizing rotational motion. The implants have a porous surface that provide an ideal environment for bone on-growth and ingrowth*, facilitating long-term fusion of the joint. iFuse is the only commercially available SI joint fusion device in the United States with published prospective clinical evidence that demonstrates safety, effectiveness and economic benefits, including three large multicenter studies, two of which are randomized controlled trials. Currently, there are more than 50 peer-reviewed publications supporting positive clinical outcomes, safety, biomechanics, and the economic value of iFuse (www.si-bone.com/results). iFuse Implant is the only SI joint fusion device with a FDA clearance recognizing demonstrated improvements in pain, patient function and quality of life following treatment.
The iFuse Implant System is intended for sacroiliac fusion for conditions including sacroiliac joint dysfunction that is a direct result of sacroiliac joint disruption and degenerative sacroiliitis. This includes conditions whose symptoms began during pregnancy or in the peripartum period and have persisted postpartum for more than 6 months. There are potential risks associated with the iFuse Implant System. It may not be appropriate for all patients and all patients may not benefit. For information about the risks, visit: www.si-bone.com/risks
* MacBarb RF, Lindsey DP, Woods SA, Lalor PA, Gundanna MI, Yerby SA. Fortifying the Bone-Implant Interface Part II: An In Vivo Evaluation of 3D-Printed and TPS-Coated Triangular Implants. Int J Spine Surg. 2017;11. [Accepted, publication pending]
SI-BONE and iFuse Implant System are registered trademarks of SI-BONE, Inc. ©2017 SI-BONE, Inc. All Rights Reserved. 9875.05012017
1 | Vanaclocha V, Herrera JM, Sáiz-Sapena N, Rivera-Paz M, Verdú-López F. Minimally Invasive Sacroiliac Joint Fusion, Radiofrequency Denervation and Conservative Management for Sacroiliac Joint Pain: Six Year Comparative Study. Neurosurgery. 2017 April 20. [Epub ahead of print]. doi: 10.1093/neuros/nyx185. |
2 | Polly DW, Swofford J, Whang PG, Frank CJ, Glaser JA, Limoni RP, Cher DJ, Wine KD, Sembrano JN, and the INSITE Study Group. Two-Year Outcomes from a Randomized Controlled Trial of Minimally Invasive Sacroiliac Joint Fusion vs. Non-Surgical Management for Sacroiliac Joint Dysfunction. Int J Spine Surg. 2016;10.Article 28. doi:10.14444/3028. |

To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/si-bone-inc-announces-si-joint-fusion-study-showed-patients-were-11x-less-likely-to-be-taking-opioids-at-last-follow-up-using-the-ifuse-implant-300448320.html
SOURCE SI-BONE, Inc.





