At the end of July 2019, the R&D pipeline contained 83 projects, including 34 new molecular entities in clinical development. 35 projects are in phase 3 or have been submitted to the regulatory authorities for approval.
PARIS, July 29, 2019 /PRNewswire/ -- Sanofi (NASDAQ: SNY; EURONEXT: SAN)
|
Q2 2019 |
Change |
Change |
H1 2019 |
Change |
Change |
|
|
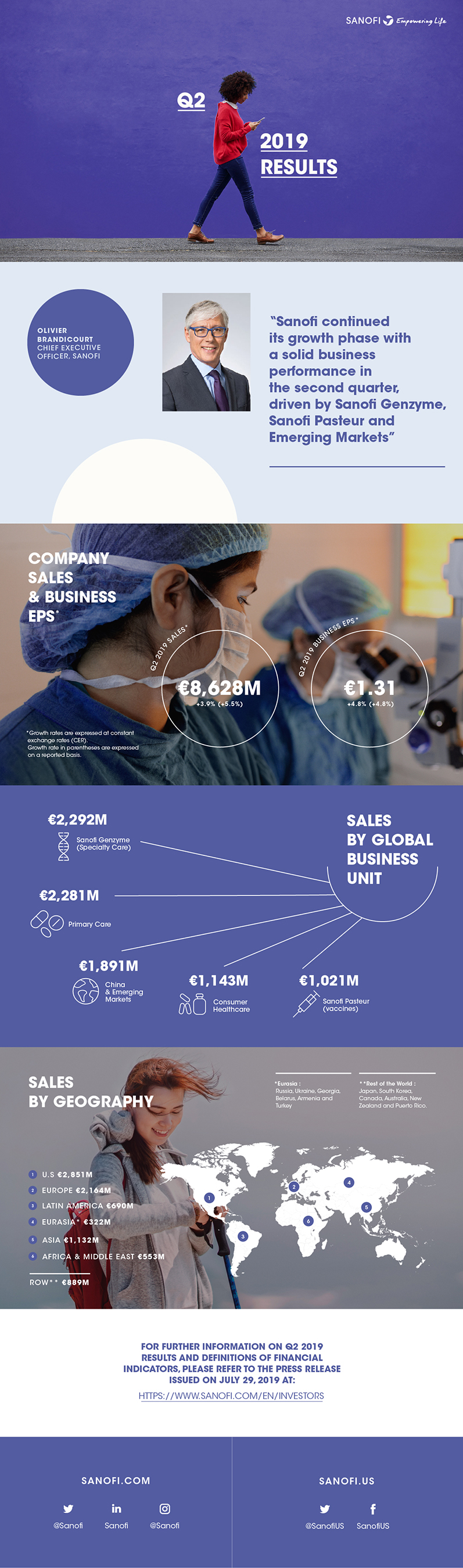
IFRS net sales reported |
€8,628m |
+5.5% |
+3.9% |
€17,019m |
+5.9% |
+4.1% |
|
IFRS net income reported |
-€87m |
-111.4%(2) |
- |
€1,050m |
-40.9% |
- |
|
IFRS EPS reported |
-€0.07 |
-111.5%(2) |
- |
€0.84 |
-40.8% |
- |
|
Business net income(1) |
€1,641m |
+5.3% |
+4.9% |
€3,406m |
+7.9% |
+7.0% |
|
Business EPS(1) |
€1.31 |
+4.8% |
+4.8% |
€2.73 |
+7.9% |
+7.1% |
Experience the interactive Multichannel News Release here: https://www.multivu.com/players/English/8581551-sanofi-earnings-results-q2-2019/
|
Second-quarter 2019 sales growth(3) driven by Sanofi Genzyme, Sanofi Pasteur and Emerging Markets
2019 business EPS guidance revised upward
Key regulatory milestones achieved in R&D
|
|
Sanofi Chief Executive Officer, Olivier Brandicourt, commented: "Sanofi continued its growth phase with a solid business performance in the second quarter, led by the strong launch of Dupixent® driven by the accelerated uptake in atopic dermatitis and asthma in the U.S. Specialty Care and Vaccines were significant contributors across all geographies. Our increased focus in R&D delivered important results with several positive data read-outs and the achievement of regulatory milestones. We are confident in the growth outlook for the year. Consequently, we have revised upward our guidance for full-year business EPS growth to approximately 5%." |
|
(1) In order to facilitate an understanding of operational performance, Sanofi comments on the business net income statement. Business net income is a non-GAAP financial measure (see Appendix 10 for definitions). The consolidated income statement for Q2 2019 is provided in Appendix 3 and a reconciliation of reported IFRS net income to business net income is set forth in Appendix 4;(2) including a €1.8 billion impairment charge mainly related to Eloctate ® – see page 12; (3) Changes in net sales are expressed at constant exchange rates (CER) unless otherwise indicated (see Appendix 10); (4) Constant Structure: Adjusted for divestment of European Generics business and sales of Bioverativ products to SOBI; (5) See definition page 9; (6) 2018 business EPS was €5.47. |
R&D update
Consult Appendix 6 for full overview of Sanofi's R&D pipeline
Regulatory update
Regulatory updates since April 26, 2019 include the following:
- In July, the U.S. Food and Drug Administration (FDA) accepted for review the Biologics License Application (BLA) for isatuximab for the treatment of patients with relapsed/refractory multiple myeloma (RRMM). The target action date for the FDA decision is April 30, 2020.
- In June, Libtayo® (cemiplimab, collaboration with Regeneron) was approved in the European Union (EU) for the treatment of adults with metastatic or locally advanced cutaneous squamous cell carcinoma (CSCC) who are not candidates for curative surgery or curative radiation.
- In June, the European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion for Dupixent® (dupilumab, collaboration with Regeneron) recommending extending its approval in the EU to include adolescents 12 to 17 years of age with moderate-to-severe atopic dermatitis who are candidates for systemic therapy.
- The FDA accepted for review the BLA for Sanofi's MenQuadfi™ Meningococcal Polysaccharide Tetanus Toxoid Conjugate Vaccine candidate to help prevent meningococcal meningitis. The target action date for the FDA decision is April 25, 2020.
- In June, the FDA approved Dupixent® for the treatment of chronic rhinosinusitis with nasal polyposis (CRSwNP) in adults whose disease is not adequately controlled.
- In May, the European Commission approved Dupixent® for use in adults and adolescents 12 years and older as an add-on maintenance treatment for severe asthma with type 2 inflammation characterized by raised blood eosinophils and/or raised fractional exhaled nitric oxide (FeNO), who are inadequately controlled with high dose inhaled corticosteroid (ICS) plus another medicinal product for maintenance treatment.
- In May, SAR341402 (insulin aspart), a rapid acting insulin, was submitted to the EMA for the treatment of Type I and II diabetes.
- In April, the FDA approved Praluent® (collaboration with Regeneron) to reduce the risk of heart attack, stroke, and unstable angina requiring hospitalization in adults with established cardiovascular disease.
At the end of July 2019, the R&D pipeline contained 83 projects, including 34 new molecular entities in clinical development. 35 projects are in phase 3 or have been submitted to the regulatory authorities for approval.
Portfolio update
Phase 3:
- Topline results from three Phase 3 trials of Zynquista™ (sotagliflozin) in adults with type 2 diabetes from the InSynchrony clinical program were announced on July 26. Given the primary endpoint results of blood sugar control (HbA1c) reduction in the SOTA-CKD3 and SOTA-CKD4 studies, Sanofi provided notice to Lexicon that it is terminating the collaboration to develop, manufacture, and commercialize Zynquista™ in all ongoing global type 1 and type 2 diabetes programs. At this time, the ongoing Phase 3 clinical trials will continue and there will be no immediate changes. Sanofi has expressed willingness to work with Lexicon to ensure a smooth transition of the studies. Sanofi remains committed to working and supporting the investigators and patients enrolled in the studies while next steps are discussed with Lexicon.
- Results from a phase 3 study evaluating Soliqua®/Suliqua® (insulin glargine 100 Units/mL and lixisenatide) in adults with type 2 diabetes inadequately controlled by GLP-1 receptor agonist (GLP-1 RA) treatments were presented at the American Diabetes Association (ADA) Scientific Sessions in June. The study met the primary objective by demonstrating a statistically superior reduction of average blood sugar level (HbA1c) after 26 weeks, compared with continuing GLP-1 RA treatment.
- Pivotal phase 3 ICARIA-MM trial results were presented at the 2019 American Society of Clinical Oncology (ASCO) Annual Meeting in June and demonstrated that isatuximab added to pomalidomide and dexamethasone (isatuximab combination therapy) showed statistically significant improvements compared to pomalidomide and dexamethasone (pom-dex) alone in patients with relapsed/refractory multiple myeloma (RRMM).
- A phase 3 study evaluating cemiplimab, a PD-1 inhibitor, in adjuvant treatment for Cutaneous Squamous Cell Carcinoma (CSCC) started.
- Dupilumab , moved into phase 3 in Chronic Obstructive Pulmonary Disease (COPD).
- Fitusiran , a siRNA inhibitor targeting AT3, entered phase 3 for pediatric hemophilia.
- Nirsevimab ( SP0232, collaboration with Medimmune), a monoclonal antibody, entered phase 3 for respiratory syncytial virus (RSV)
Phase 2:
- SAR440340/REGN3500 (collaboration with Regeneron), an investigational IL-33 antibody, met the primary endpoint of improvement in loss of asthma control when comparing monotherapy to placebo in a phase 2 proof-of-concept trial The trial also met a key secondary endpoint, demonstrating SAR440340 monotherapy significantly improved lung function compared to placebo. Patients treated with Dupixent® monotherapy did numerically better than SAR440340 across all endpoints, although the trial was not powered to show differences between active treatment arms. The combination of SAR440340 and Dupixent® did not demonstrate increased benefit compared to Dupixent® monotherapy in this trial.
Phase 1:
- A phase 1 trial evaluating SAR441255, a trigonal GLP1R/GIPR/GCGR agonist was initiated.
- SAR441236 , a tri-specific neutralizing anti-HIV mAb, entered into phase 1.
An additional seven research projects have been discontinued to enhance the company's focus on delivering first and best in class medicines
Collaboration
In June, Sanofi and Google announced that they will establish a new virtual Innovation Lab with the ambition to transform how future medicines and health services are delivered by tapping into the power of emerging data technologies. The collaboration aims to change how Sanofi develops new treatments and will focus on three key objectives: to better understand patients and diseases, to increase Sanofi's operational efficiency, and to improve the experience of Sanofi's patients and customers.
To access the full press release of the 2019 Q2 results, please click here.
Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These statements include projections and estimates and their underlying assumptions, statements regarding plans, objectives, intentions and expectations with respect to future financial results, events, operations, services, product development and potential, and statements regarding future performance. Forward-looking statements are generally identified by the words "expects", "anticipates", "believes", "intends", "estimates", "plans" and similar expressions. Although Sanofi's management believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Sanofi, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include among other things, the uncertainties inherent in research and development, future clinical data and analysis, including post marketing, decisions by regulatory authorities, such as the FDA or the EMA, regarding whether and when to approve any drug, device or biological application that may be filed for any such product candidates as well as their decisions regarding labelling and other matters that could affect the availability or commercial potential of such product candidates, the absence of guarantee that the product candidates if approved will be commercially successful, the future approval and commercial success of therapeutic alternatives, Sanofi's ability to benefit from external growth opportunities, to complete related transactions and/or obtain regulatory clearances, risks associated with intellectual property and any related pending or future litigation and the ultimate outcome of such litigation, trends in exchange rates and prevailing interest rates, volatile economic conditions, the impact of cost containment initiatives and subsequent changes thereto, the average number of shares outstanding as well as those discussed or identified in the public filings with the SEC and the AMF made by Sanofi, including those listed under "Risk Factors" and "Cautionary Statement Regarding Forward-Looking Statements" in Sanofi's annual report on Form 20-F for the year ended December 31, 2018. Other than as required by applicable law, Sanofi does not undertake any obligation to update or revise any forward-looking information or statements.
|
Media Relations: |
Investor Relations: |
||
|
Ashleigh Koss |
George Grofik |
||
|
908-981-8745 |
+33 (0)1 53 77 45 45 |
||
|
Email: Ashleigh.koss@sanofi.com |
Email: IR@sanofi.com |



![]() View original content:http://www.prnewswire.com/news-releases/sanofi-delivered-solid-growth-in-q2-2019-300892206.html
View original content:http://www.prnewswire.com/news-releases/sanofi-delivered-solid-growth-in-q2-2019-300892206.html
SOURCE Sanofi
Company Codes: NASDAQ-NMS:SNY, EuronextParis:SAN




