Wellysis, a spin-off company from Samsung SDS, has received clearance from the U.S. Food and Drug Administration (FDA) on August 30, 2023, to market its wearable electrocardiography (ECG) patch, S-Patch Ex, in the U.S.Wellysis, a spin-off company from Samsung SDS, has received clearance from the U.S. Food and Drug Administration (FDA) on August 30, 2023, to market its wearable electrocardiography (ECG) patch, S-Patch Ex, in the U.S.
|
|
| [13-September-2023] |
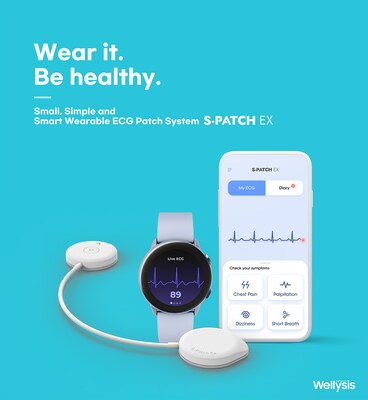
SEOUL, South Korea, Sept. 13, 2023 /PRNewswire/ -- Wellysis, a spin-off company from Samsung SDS, has received clearance from the U.S. Food and Drug Administration (FDA) on August 30, 2023, to market its wearable electrocardiography (ECG) patch, S-Patch Ex, in the U.S. The product, which received EU's CE mark in May 2021, is currently being exported to 14 countries worldwide, including UK, Spain, Italy, Greece, Poland, Australia, Thailand, India, and Korea. The S-Patch Ex is an ECG and heart rate recording device for use by healthcare professionals and patients both at home and in clinical settings. The product is embedded with Samsung Electronics' Next-Generation bio-processor which ensures the device captures accurate ECG waveforms. The product is expected to be officially launched in the U.S. market in September by establishing partnerships with service and platform providers. With the latest technologies on board, the performance and features of the product are unparallel to those of key players in the wearable patch market. Weighing just 9g, S-Patch Ex offers the market's lightest weight, continuous test duration, and integrates with mobile phones, tablets, and watches, as well as compatible with any commercially available electrodes and batteries. Furthermore, the product is reusable and equipped with waterproof and dustproof features, boasting an IP55 rating, ensuring durability in various conditions. "S-Patch Ex is designed with patience-centric for approach to ensure their comfort and ease of use in most daily activities. It also provides the most accurate information to the physicians. We are excited to introduce its outstanding features to the US market base with the FDA clearance in the U.S.," said Young Juhn, CEO and Founder of Wellysis. S-Patch Ex is used predominantly on patients with symptoms such as palpitations. Most recently, the device is also used in research with long COVID patients for ECG monitoring in institutions such as Stanford University in the U.S. and Liverpool Hospital in Australia. About Wellysis Wellysis is a South Korean startup founded in 2019 as a spin-off from Samsung SDS's Digital Health division by a team of experts with extensive experience in the healthcare field. The company has attracted investment from major pharmaceutical companies and investors, with a total of 12 million USD up to date. In just five years since its establishment, the company has successfully expanded its business to overseas markets, with over 790 hospitals and clinics using the product worldwide.
SOURCE Wellysis |