PRP a Potential Method for Reducing Recurrence After Drug Treatment Failures Global Metastatic Cancer Treatment Market Anticipated to Reach $98.24 Billion By 2025
MELBOURNE, Australia--(BUSINESS WIRE)-- Propanc Biopharma, Inc. (OTC: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing new cancer treatments for patients suffering from recurring and metastatic cancer, announced today that the mode of action of the company’s lead product candidate, PRP, a formulation consisting of two proenzymes, has been elucidated by the company’s researchers, by suppressing pathways relating to the Epithelial to Mesenchymal Transition (EMT) and metastasis. The EMT is a biological process by which cells become motile and invasive, but in cancer stem cells (CSCs), results in metastasis, a process whereby secondary tumors are formed often in remote distances from a primary tumor, causing the cancer to return and spread. Scientific data relating to these key findings were published in Scientific Reports, an online open access journal from the Publishers of Nature, entitled, “Pancreatic Proenzymes treatment suppresses BXPC-3 pancreatic Cancer Stem Cell subpopulation and impairs tumor engrafting.”
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20191021005232/en/
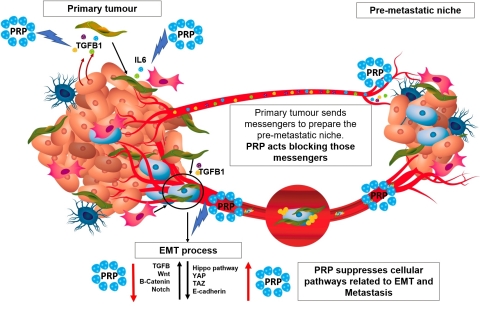
PRP mode of action (Graphic: Business Wire)
“Our findings from our recently published paper confirms the mechanism of action of PRP, which may prove to be effective tool in the fight against metastatic cancer, the main cause of patient death for sufferers,” said Dr Julian Kenyon, Propanc’s Chief Scientific Officer. “By targeting CSCs, PRP is targeting the fundamental mechanism by which cancer spreads, especially after exposure to chemo or radiation, because they are non-dividing cells and are therefore resistant to standard treatments. The mode of action of PRP is complex due to the biological nature of the proenzymes and its multiple modes of action. By defining how it works, it gives us confidence that this treatment method has potential implications in a clinical setting, for example, by reducing recurrence after drug treatment failures in cancer patients.”
CSCs have been characterized as immortal cells that grow inside tumours and have a great tumour-initiating capacity. Disturbingly, CSCs have a capacity to remain dormant that make them radio- and chemo-resistant, whilst highly metastatic. As cancer treatment moves towards more personalised medicine targeting specific genes, proper therapies to target and treat specifically CSCs are needed for reducing recurrence after drug resistance failures.
Recently, an exhaustive scientific study with the company’s joint researchers proved that PRP dramatically impairs the maintenance of CSCs characteristics, eradicating these malignant cell populations, even under cell culture conditions that support their enrichment and growth.
The recent study addresses, in depth, the effect of PRP on the cellular mechanism characteristic of pancreatic CSCs. The modulation of hundreds of genes after treatment were analysed and proved the high impact that PRP has on the CSCs population. In fact, the treatment inhibited the expression of genes related to CSCs characteristics, which means that PRP was able to change the nature of these malignant cells toward a more differentiated and less dangerous cellular condition.
From a more detailed scientific perspective, PRP treatment regulates up to four pathways related to cancer spread and metastasis, such as TGFβ, Hippo, Wnt and Notch pathways. The cascade of reactions that PRP imposes on tumour cells, implies the disruption of the CSC characteristics, which reverses the malignant EMT process that leads to tumour invasion.
Furthermore, PRP downregulation of TGFβ-1, had implications in tumour engraftment. In vivo experiments using a nude mice model, in which tumours were induced by inoculation of pancreatic CSCs, showed that PRP impaired CSCs subpopulation activation, niche formation and tumour initiation. In others words, PRP treatment seems to have a preventive effect against cancer. CSCs that were inoculated after treatment with PRP did not find a “comfortable surrounding” where to nest, implying the anticancer potential of this novel drug.
“We continue to make significant progress with our scientific understanding and development of our lead product, PRP, as a targeted cancer stem cell therapy. The global metastatic cancer treatment market is predicted to reach nearly $100 Billion over the next 5-year period, and we believe PRP has the potential to impact the way we view and treat cancer, by minimizing the potential for its return and spread in patients. We have recently completed preclinical development and look forward to advancing PRP into clinical trials in the near future,” said James Nathanielsz, Propanc’s Chief Executive Officer.
According to a new market intelligence report by BIS Research, titled "Global Metastatic Cancer Treatment Market – Analysis and Forecast, 2018-2025", the global metastatic cancer treatment market was estimated at $54.11 billion in 2017, and anticipated to reach $98.24 billion by 2025.
About Propanc Biopharma, Inc.
Propanc Biopharma, Inc. (the “Company”) is developing a novel approach to prevent recurrence and metastasis of solid tumors by using pancreatic proenzymes that target and eradicate cancer stem cells in patients suffering from pancreatic, ovarian and colorectal cancers. For more information, please visit www.propanc.com.
The Company’s novel proenzyme therapy is based on the science that enzymes stimulate biological reactions in the body, especially enzymes secreted by the pancreas. These pancreatic enzymes could represent the body’s primary defense against cancer.
To view the Company’s “Mechanism of Action” video on its anti-cancer lead product candidate, PRP, please click on the following link: http://www.propanc.com/news-media/video
Forward-Looking Statements
All statements other than statements of historical facts contained in this press release are “forward-looking statements,” which may often, but not always, be identified by the use of such words as “may,” “might,” “will,” “will likely result,” “would,” “should,” “estimate,” “plan,” “project,” “forecast,” “intend,” “expect,” “anticipate,” “believe,” “seek,” “continue,” “target” or the negative of such terms or other similar expressions. These statements involve known and unknown risks, uncertainties and other factors, which may cause actual results, performance or achievements to differ materially from those expressed or implied by such statements. These factors include uncertainties as to the Company’s ability to continue as a going concern absent new debt or equity financings; the Company’s current reliance on substantial debt financing that it is unable to repay in cash; the Company’s ability to successfully remediate material weaknesses in its internal controls; the Company’s ability to reach research and development milestones as planned and within proposed budgets; the Company’s ability to control costs; the Company’s ability to obtain adequate new financing on reasonable terms; the Company’s ability to successfully initiate and complete clinical trials and its ability to successful develop PRP, its lead product candidate; the Company’s ability to obtain and maintain patent protection; the Company’s ability to recruit employees and directors with accounting and finance expertise; the Company’s dependence on third parties for services; the Company’s dependence on key executives; the impact of government regulations, including FDA regulations; the impact of any future litigation; the availability of capital; changes in economic conditions, competition; and other risks, including, but not limited to, those described in the Company’s Registration Statement on Form S-1, Amendment No. 1, filed with the U.S. Securities and Exchange Commission (the “SEC”) on June 14, 2019, and in the Company’s other filings and submissions with the SEC. These forward-looking statements speak only as of the date hereof and the Company disclaims any obligations to update these statements except as may be required by law.
View source version on businesswire.com: https://www.businesswire.com/news/home/20191021005232/en/
Contacts
Investor Relations and Media:
Carlo Campiciano
Propanc Biopharma, Inc.
irteam@propanc.com
+61-03-9882-6723
Source: Propanc Biopharma, Inc.