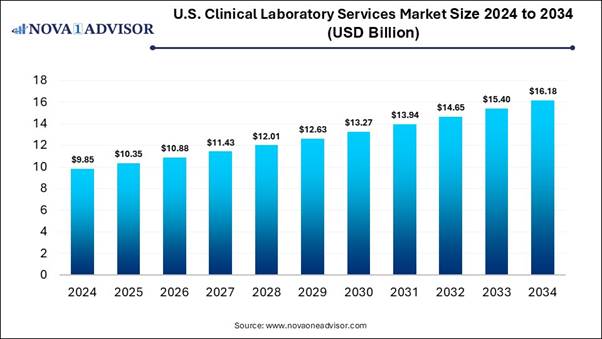
According to Nova One Advisor, the U.S. clinical laboratory services market size is expected to be worth around 16.18 billion by 2034, increasing from USD 10.35 billion in 2025, representing a healthy CAGR of 16.18% from 2025 to 2034.
The U.S. clinical laboratory services market is growing due to these laboratory services significantly providing precise treatment planning, diagnosis, and monitoring health people. These laboratory professionals offer clinical data and services that contribute to the efficient delivery of care in the complex medical care system. U.S. clinical laboratories serve as the foundation for public health readiness and response, as well as for everyday diagnosis, prevention, and treatment of disease. Well-organized lab outcomes delivery not only speeds up diagnoses but also reduces errors and optimizes workflows. Laboratory services in hospitals play a significant role in the identification and diagnosis of infection.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report@ https://www.novaoneadvisor.com/report/sample/9202
U.S. Clinical Laboratory Services Market Highlights:
• By test type, the clinical chemistry segment dominated the market with the largest share in 2024.
• By test type, the cytology testing segment is expected to show the fastest growth over the forecast period.
• By application, the bioanalytical & lab chemistry services segment held the largest market share in 2024.
• By application, the toxicology testing services segment is expected to register fastest growth during the forecast period.
• By service provider, the hospital-based laboratories segment captured the largest market revenue share in 2024.
• By service provider, the stand-alone laboratories segment is expected to show the fastest growth during the forecast period.
Market Overview and Industry Potential
Clinical laboratories are medical care facilities offering a broad range of laboratory techniques that aid clinicians in identifying, treating, and managing patients. Clinical laboratories employ pathologists, lab biochemists, laboratory assistants, laboratory managers, biomedical scientists, therapeutic laboratory technicians, health laboratory assistants, phlebotomists, and histology technicians. Clinical laboratory authorities perform various tasks, involving advancing and validating novel laboratory tests, evaluating and crucial the analytical and clinical performance, assigning laboratory outcomes to clinicians, providing valuable education and guidance to the medical team, assessing the affordable and intrinsic value.
Clinical laboratories are seeing the growing adoption of automation and artificial intelligence. These systems, primarily deployed during the COVID-19 pandemic to handle the surge in testing volumes, are presently being used to streamline different laboratory procedures. These systems lower manual steps, enhance the reproducibility of results, and improve the overall effectiveness of laboratories. AI, on the other hand, is transforming diagnostics by allowing rapid and more precise analysis of complex information. AI-driven devices are predominantly beneficial in areas like pathology, where they assist in the interpretation of microscopic images and the identification of irregularities.
⬥︎ For Instance, In May 2025, Elegen, the leader in next-generation cell-free DNA manufacturing, announced the early access launch of ENFINIA IVT Ready DNA. An expansion of its ENFINIA platform, this new product is delivered ready-to-use with the requisite poly(A) tail already encoded.
Latest Trends of the Market
⬥︎ In February 2025, the Association for Diagnostics & Laboratory Medicine (ADLM, formerly AACC) released a position statement emphasizing that limited access to quality clinical laboratory services is impeding children’s health, and that action must be taken to address this significant problem. In particular, the statement calls on Congress to increase funding for programs such as the Children’s Health Insurance Program and newborn screening to create a more equitable healthcare landscape for children.
⬥︎ In April 2025, the National Accreditation Board for Testing and Calibration Laboratories (NABL), a constituent board of the Quality Council of India (QCI), today launched its new Medical Application Portal designed for ISO 15189:2022 applicant laboratories. The online portal was unveiled during a virtual event titled “GOING LIVE”, marking a major step in NABL’s mission to enhance ease, efficiency, and transparency in the accreditation process.
Increasing Adoption of Next-generation Sequencing (NGS): Market’s Largest Potential
Next-generation sequencing (NGS) is a ground-breaking novelty transforming clinical laboratories. NGS enables the fast and comprehensive analysis of genetic material, offering insights into the genetic basis of diseases and allowing targeted medicine. This technology is specifically helpful in oncology, where it supports identifying genetic mutations that drive cancer progression and data-targeted treatment approaches. The integration of NGS in the routine clinical practice has led to the advancement of developed diagnostic tests that detect a broad range of genetic disorders. These tests are becoming progressively accessible, with advancements in sequencing technology and cost reductions. NGS is poised to become a standard device in clinical laboratories, providing unprecedented chances for early disease identification and personalized treatment.
⬥︎ For Instance, In August 2025, BioMark Diagnostics Inc. announced the completion of a strategic laboratory equipment leasing agreement, substantially doubling BioMark’s testing capacity and positioning the Company for the imminent commercial launch of its lung cancer assay. This critical advancement in its operational capabilities solidifies the expansion of its diagnostic and research services and positions the Company as a central hub for innovation, high-throughput diagnostics, and collaborative ventures.
Buy Now Full Report: https://www.novaoneadvisor.com/report/checkout/9202
Report Scope of U.S. Clinical Laboratory Services Market
|
Report Coverage |
Details |
|
Market Size in 2025 |
USD 10.35 Billion |
|
Market Size by 2034 |
USD 16.18 Billion |
|
Growth Rate From 2025 to 2034 |
CAGR of 5.09% |
|
Base Year |
2024 |
|
Forecast Period |
2025-2034 |
|
Segments Covered |
By Test Type, By Service Provider, By Application |
|
Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
|
Key Companies Profiled |
ARUP Laboratories, Charles River Laboratories International, Inc., Eurofins Scientific SE, Fresenius Medical Care, Laboratory Corporation of America Holdings (LabCorp), Mayo Clinic Laboratories, NeoGenomics Laboratories, OPKO Health, Inc., Siemens Medical Solutions USA, Inc., Sonic Healthcare, SYNLAB International GmbH, QIAGEN NV, Quest Diagnostics Incorporated, Unilabs |
U.S. Clinical Laboratory Services Market
Segmentation Analysis: By Test Type: The clinical
chemistry segment dominates in the U.S. clinical laboratory services
market, as it has the ability to offer accurate and precise measurements of
biochemical materials in biological fluids. This supports the early detection
of diseases, which is important for efficient management and treatment. It
plays a significant role in current medicine. It is used for the monitoring and
diagnosis of diseases, the resolution of drug levels and efficacy, and the
identification of toxic substances in the body. On the other hand, the cytology testing
segment is expected to grow expressively during the forecast period as it is a
crucial diagnostic device that supports identifying and monitor a broad range
of diseases, from cancer to infections and inflammatory conditions. It is a
minimally invasive technology that offers appreciated insights into the health
of individual cells, helping doctors make informed decisions related to
diagnosis and treatment. Cytology involves the collection of cell samples from
the body, tracked by their analysis in a microscope. By Application Analysis: The bioanalytical & lab chemistry
services segment dominated the U.S. clinical laboratory services market, as
using bioanalytical
services provides many benefits, containing access to advanced
analytical processes, enhanced data accuracy, and compliance with regulatory
standards. These services allow researchers to gain deeper insights into the
behavior of drug candidates, important to more informed decision-making and
growing chances of success in medical development. Bioanalytical services
support to development of more effective production processes, whether
operating in the biotechnology, pharmaceutical, or chemistry fields. The toxicology testing segment is expected
to grow significantly during the forecast period as this testing plays a
significant role in detecting the potential adverse events caused by chemicals.
Genotoxicity, carcinogenicity, reproductive, immunotoxicity, and developmental
toxicity in humans are usually observed after chronic chemical exposure.
Toxicologic screening supports to guidance of severe management or chronic
treatment. By Service Provider Analysis: The hospital-based laboratories segment
accounted for the largest market share in 2024, as these laboratories are
staffed by a team of trained laboratory specialists, including healthcare
technologists, laboratory pathologists, and technicians. These specialists are
responsible for conducting different laboratory tests, such as blood tests. Its
ability to provide a quick turnaround time for test results. These labs allow
healthcare providers to admittance test data proximately, enabling quicker
decision-making concerning patient care and treatment plans. On the other hand, the stand-alone
laboratories segment is expected to grow significantly during the forecast
period as this laboratory system needs regular manual data backups. It is not
allowed to manage with unexpected disasters or pandemics. The stand-alone
laboratory is a station part that has all the necessary equipment to serve as a
functional space station. Accessibility of a stand-alone laboratory is feasible
on a single computer/laptop, which would be in a lab environment. U.S. Clinical Laboratory Services Market
Key Regional Analysis: The United States is a clinical laboratory
tests saves time, expenses, and lives by allowing early detection and
prevention of disease. Greater than 7 billion clinical lab tests are performed
in the U.S. every year, offering critical data for a relatively small cost.
U.S. clinical labs help hundreds of thousands of employees, with a skilled
professional comprised of pathologists’ assistants, medical laboratory
scientists, biochemists, phlebotomists, pathologists, and other highly skilled
medical staff. With 80% of the nation’s 322,488 clinical laboratories working
as small businesses, the subdivision is a noteworthy contributor to local jobs
and economies. ⬥︎ For Instance, 2023, In July 2025, the
American Clinical Laboratory Association (ACLA) today announced that Laura
Stevens Kent has joined the organization as Senior Vice President, Government
Affairs & Policy. Stevens Kent brings two decades of experience in federal
and state advocacy, organizational strategy, and health care policy to the
role. Immediate Delivery is Available | Get
Full Report Access@ https://www.novaoneadvisor.com/report/checkout/9202
Value Chain Analysis Diagnostic Information Services These services refer to a broad range of
medical tests, imaging scans, and procedures utilized for identification
illnesses and for determining their severity, which enables accurate diagnosis,
treatment planning and management of diseases, playing an important role in
both patient care and preventive medicine. Key Players: • BioReference Health, LLC • Quest Diagnostics • LabCorp 2. Patient Support and Services These services comprise of specimen
collection, interpretation of results, and communication with patients and
their healthcare providers. Key Players: • ARUP Laboratories • NeoGenomics Laboratories 3. R&D Research and development focuses on enhancing
the accuracy, efficiency, and speed of clinical trials, as well as improving
data interpretation and decision-making, further leading to faster and more
reliable clinical R&D workflows. Key Players: • Abbott Laboratories, • Charles River Laboratories • IQVIAThermo Fisher Scientific U.S. Clinical Laboratory Services Market
Companies: • ARUP Laboratories • Charles River Laboratories International,
Inc. • Eurofins Scientific SE • Fresenius Medical Care • Laboratory Corporation of America Holdings
(LabCorp) • Mayo Clinic Laboratories • NeoGenomics Laboratories • OPKO Health, Inc. • Siemens Medical Solutions USA, Inc. • Sonic Healthcare • SYNLAB International GmbH • QIAGEN NV • Quest Diagnostics Incorporated • Unilabs What is Going Around the Globe? ⬥︎ In January 2025, Quest Diagnostics, a
leading provider of diagnostic information services, announced it had completed
its previously announced acquisition of select assets of University Hospitals,
one of the nation's leading nonprofit health systems and academic medical
centers. Financial terms were not disclosed. ⬥︎ In March 2025, IQVIA Laboratories, a
leading global drug discovery and development laboratory services organization,
announced the launch of Site Lab Navigator, an advanced suite of solutions that
automates and streamlines lab workflows for clinical trial sponsors and
investigator sites. ⬥︎ In February 2025, Illumina, Inc., a
global leader in next-generation sequencing and array-based technologies,
announced a collaboration with Broad Clinical Labs to rapidly streamline and
scale single-cell projects with cutting-edge tools and workflows. You can place an order or ask any
questions, please feel free to contact at sales@novaoneadvisor.com |
+1 804 441 9344 Related Report – ⬥︎ Latin America Clinical Laboratory Services Market - https://www.novaoneadvisor.com/report/latin-america-clinical-laboratory-services-market
⬥︎ Asia Pacific Clinical Laboratory Services Market - https://www.novaoneadvisor.com/report/asia-pacific-clinical-laboratory-services-market
⬥︎ Europe Clinical Laboratory Services Market - https://www.novaoneadvisor.com/report/europe-clinical-laboratory-services-market
⬥︎ Direct-to-Consumer Laboratory Testing Market - https://www.novaoneadvisor.com/report/direct-to-consumer-laboratory-testing-market
⬥︎ Clinical Laboratory Tests Market - https://www.novaoneadvisor.com/report/clinical-laboratory-tests-market
⬥︎ U.S. Laboratory Automation Market - https://www.novaoneadvisor.com/report/us-laborator-automation-market
Segments Covered in the Report This report forecasts revenue growth at
country levels and provides an analysis of the latest industry trends in each
of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc.
has segmented the U.S. Clinical Laboratory Services Market. By Test Type • Genetic Testing • Clinical Chemistry ° Routine
Chemistry Testing ° Therapeutic Drug
Monitoring Testing ° Endocrinology
Chemistry Testing ° Specialized
Chemistry Testing ° Other Clinical
Chemistry Testing • Medical Microbiology Testing ° Infectious
Disease Testing ° Transplant
Diagnostic Testing ° Other
Microbiology Testing • Hematology Testing • Immunology Testing • Cytology Testing • Drug of Abuse Testing • Other Esoteric Tests By Service Provider • Clinic-Based Laboratories • Hospital-Based Laboratories • Stand-Alone Laboratories By Application • Bioanalytical & Lab Chemistry
Services • Cell & Gene Therapy Related Services • Drug Discovery & Development Related
Services • Preclinical & Clinical Trial Related
Services • Toxicology Testing Services • Other Clinical Laboratory Services Immediate Delivery
Available | Buy This Premium Research https://www.novaoneadvisor.com/report/checkout/9202 About-Us Nova One Advisor is a global leader
in market intelligence and strategic consulting, committed to delivering deep,
data-driven insights that power innovation and transformation across
industries. With a sharp focus on the evolving landscape of life sciences, we
specialize in navigating the complexities of cell and gene therapy, drug
development, and the oncology market, enabling our clients to lead in some of
the most revolutionary and high-impact areas of healthcare. Our expertise spans the entire
biotech and pharmaceutical value chain, empowering startups, global
enterprises, investors, and research institutions that are pioneering the next
generation of therapies in regenerative medicine, oncology, and precision
medicine. Web: https://www.novaoneadvisor.com/ Contact Us USA: +1 804 420 9370 Email: sales@novaoneadvisor.com For Latest Update Follow Us: LinkedIn

