According to Nova One Advisor, the U.S. antiviral drugs market size is expected to be worth around 21.94 billion by 2034, increasing from USD 26.31 billion in 2025, representing a healthy CAGR of -2.0% from 2025 to 2034.
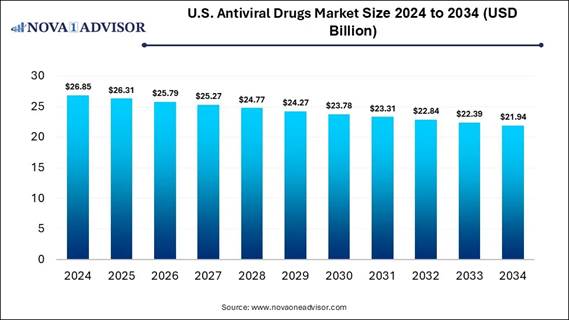
The U.S. antiviral drugs market is expanding as the increasing prevalence of viral diseases, recent advancements in research and development of antiviral therapy, rising awareness and diagnosis of viral infection, and a growing aging population, they are more susceptible to viral diseases. Every antiviral drug only works against an exact virus. This is because viruses in cells are challenging to target, development of antiviral drugs is a more complex process. There are require viruses than antiviral drugs to treat them.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report@ https://www.novaoneadvisor.com/report/sample/9193
U.S. Antiviral Drugs Market Highlights:
• By type, the branded antiviral drugs segment dominated the market with a revenue share in 2024.
• By type, the generic antiviral drugs segment is expected to grow at the fastest CAGR in the market during the forecast period.
• By drug class, the reverse transcriptase inhibitors (RTIs) segment led the market with the largest revenue share in 2024.
• By drug class, the DNA polymerase inhibitors segment is expected to grow at the fastest CAGR in the market during the forecast period.
• By application, the HIV segment held the largest market share in 2024.
• By application, the herpes segment is expected to grow at the fastest CAGR in the market during the forecast period.
• By distribution channel, the hospital pharmacy segment held the highest market share in 2024.
• By distribution channel, the online pharmacy segment is expected to grow at the fastest CAGR in the market during the forecast period.
Market Overview and Industry Potential
Antiviral drugs are a class of medications predominantly applied for the treatment of viral infections. Viruses are significant pathogenic agents that cause several numbers of severe diseases in plants, animals, and humans. Novel progression strategies for the antiviral drugs are focused on two approaches such as targeting the viruses themselves and the host cell factors.
Antiviral drugs work against both types of viruses, such as DNA viruses and RNA viruses. Major antiviral drugs are acyclovir, valacyclovir, penciclovir, famciclovir, foscarnet, ribavirin, lamivudine and amantadine and rimantadine.
⬥︎ For Instance, In June 2025, Atea Pharmaceuticals, Inc., announced that the first patient was dosed in the global Phase 3 C-FORWARD trial evaluating the combination regimen of bemnifosbuvir and ruzasvir compared to the regimen of sofosbuvir and velpatasvir for the treatment of hepatitis C virus (HCV). C-FORWARD, the second of two Phase 3 trials comparing this regimen, is being conducted at study sites outside of North America.
Viral diseases are continuously increasing in the U.S., which is an increasing burden on the health care system and health-care resources. Endemic diseases like chronic hepatitis, HIV, and other sexually transmitted infections affect millions of U.S. people and exacerbate health disparities. Furthermore, challenges include healthcare-associated and foodborne infections, which are increasing the demand for antiviral drugs.
What are Latest Trends of the U.S. Antiviral Drugs Market?
⬥︎ In June 2025, Gilead Sciences, Inc. announced that the U.S. Food and Drug Administration (FDA) approved Yeztugo the company’s injectable HIV-1 capsid inhibitor as pre-exposure prophylaxis (PrEP) to lowers the risk of sexually acquired HIV in adults and adolescents weighing at least 35kg, making it the first and only twice-yearly option available in the United States for people who need or want PrEP.
⬥︎ In July 2025, ViiV Healthcare, the worldwide specialist HIV company majority owned by GSK, with Pfizer and Shionogi as shareholders, announced their voluntary licensing agreement with the Medicines Patent Pool (MPP) for cabotegravir to include patents relating to its use in a long-acting HIV treatment regimen. The announcement follows updated guidance from WHO recommending long-acting injectable cabotegravir + rilpivirine as an HIV treatment option.
Recent Advancements of Combination Therapy for the Viral Diseases: Market’s Largest Potential
Recent advancements in combination therapy for viral diseases have led to enhanced clinical results with dramatic decreases in viral load, mortality, and morbidity. Drug combinations therapy improves therapeutic effectiveness by additive, and preferably synergistic, effects for emerging and re-emerging viruses, like influenza, severe acute respiratory syndrome-coronavirus (SARS-CoV), Middle East respiratory syndrome (MERS-CoV)-CoV, Ebola, Zika, and SARS-coronavirus 2 (CoV-2).
Antiviral combinations increase the treatment spectrum, making them more efficient against a larger range of viruses, including emerging variants. For instance, Paxlovid, a combination of nirmatrelvir and ritonavir, lowers the risk of hospitalization and death in mild to moderate cases when it is given in the first few days of infection.
Buy Now Full Report: https://www.novaoneadvisor.com/report/checkout/9193
Report Scope of U.S. Antiviral Drugs Market
|
Report Coverage |
Details |
|
Market Size in 2025 |
USD 26.31 Billion |
|
Market Size by 2034 |
USD 21.94 Billion |
|
Growth Rate From 2025 to 2034 |
CAGR of -2% |
|
Base Year |
2024 |
|
Forecast Period |
2025-2034 |
|
Segments Covered |
Type, Drug Class, Application, Distribution Channel |
|
Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
|
Key Companies Profiled |
F. Hoffmann-La Roche Ltd., AbbVie, Inc., Merck & Co. Inc., Johnson & Johnson Services, Inc., Bristol-Myers Squibb Company, Cipla Inc., Aurobindo Pharma, Dr. Reddy’s Laboratories Ltd., ADARx Pharmaceuticals Inc. |
U.S. Antiviral Drugs Market Segmentation Analysis:
By Type Analysis:
The branded antiviral drugs segment dominates in the U.S. antiviral drugs market, as brand antiviral drugs are discoveries developed by research and clinical trials. Novel drugs go through many years of testing on animals and humans to demonstrate they are safe and effective, earlier they are ready for usage. This takes a lot of money, so brand healthcare organizations get patent and exclusivity protection from competition for numerous years.
On the other hand, the generic antiviral drugs segment is expected to grow exponentially during the forecast period as these drugs offer important advantages, like affordability, rising accessibility, therapeutic equivalence, and reliable quality and safety. By selecting generic drugs, patients can access affordable management of viral infection instead of compromising on effectiveness or safety. The availability of generic drugs increases management options and contributes to the overall cost-effectiveness of medical care systems.
By Drug Class Analysis:
The reverse transcriptase inhibitors (RTIs) segment generated the highest market revenue in 2024, as they help lower the outbreak of HIV from mother to child during pregnancy and labor and delivery. The drug of choice for HIV treatment of the mother during pregnancy is zidovudine-based antiretroviral therapy. The class of reverse transcriptase inhibitors is divided into two subclasses of drugs such as nucleoside/nucleotide reverse transcriptase inhibitors and non-nucleoside reverse transcriptase inhibitors.
On the other hand, the DNA polymerase inhibitors segment is expected to grow exponentially during the forecast period as DNA polymerases are required to precisely and effectively replicate the genome in order to ensure the preservation of the genetic data and its correct transmission through generations. These drugs target the virus's duplication by interfering with the action of the virus's precise polymerases. In this respect, they also act as obligate or as non-obligate chain terminators, which in both cases lastly cause partial replication of the viral genome and thus produce incompetent new virus particles.
By Application Analysis:
The HIV segment generated the highest market revenue in 2024, as antiretroviral therapy uses a combination of medications to treat HIV. It works by stopping HIV from replicating. It lowers levels of HIV and keeps the immune system strong. This treatment is not completely curative, but it offers longer lives for patients and reduces HIV transmission.
On the other hand, the herpes segment is expected to grow exponentially during the forecast period as antiviral drugs support to control the symptoms and limit the duration of initial herpes occurrences by 2 to 4 days. These drugs are taken in tablet form. Systemic antiviral drugs moderately control the signs and symptoms of genital herpes when applied to treat the first clinical and recurrent episodes.
By Distribution Channel Analysis:
The hospital pharmacy segment generated the highest market revenue in 2024, as it rising interaction with prescribers and other health specialists. It offers greater input in prescribing decisions related to drugs and administration. This pharmacy includes a larger team of pharmacists working in the same institution. It provides access to medical records of patients and has a patient-centric focus in pharmacy operations, leading to more effective hospital operations, which in turn enhance patient experience.
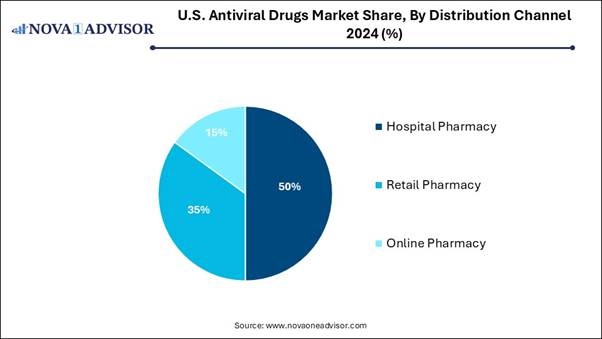
On the other hand, the online pharmacy segment is expected to grow exponentially during the forecast period, as this pharmacy provides many advantages, mainly because it is timesaving. Consumer orders medication within minutes and gets prescription medicines. This pharmacy also offers great convenience, particularly for those patients who are afraid of talking directly with doctors and pharmacists. It provides a broader range of options than a traditional pharmacy.
Country Level Analysis:
In 2024, a total of 889 cases and 463 deaths caused by the influenza A(H5N1) virus in the U.S., which is increasing demand for the antiviral drugs market. Drug discovery in the U.S. is experiencing a groundbreaking transformation driven by innovative technologies. From AI and machine learning accelerating antiviral drug candidate identification to CRISPR gene editing allowing accurate interventions, and mRNA platforms are accelerating the antiviral discovery and development.
⬥︎ For Instance, In June 2025, AbbVie announced that the U.S. Food and Drug Administration (FDA) approved a label expansion for MAVYRET, an oral pangenotypic direct-acting antiviral (DAA) therapy. It is approved for the treatment of adults and pediatric patients three years and older with acute or chronic hepatitis C virus (HCV) infection without cirrhosis or with compensated cirrhosis. With this approval, MAVYRET is the first and only DAA therapy approved to treat patients with acute HCV in eight weeks with a 96% cure rate.
U.S. Antiviral Drugs Market Companies:
• F. Hoffmann-La Roche Ltd.
• AbbVie, Inc.
• Johnson & Johnson Services, Inc.
• Bristol-Myers Squibb Company
• Cipla Inc.
• Dr. Reddy’s Laboratories Ltd.
What is Going Around the Globe?
⬥︎ In February 2025, Gilead Sciences, Inc. announced that the U.S. Food and Drug Administration (FDA) had accepted its New Drug Application (NDA) submissions for lenacapavir, the company’s twice-yearly injectable HIV-1 capsid inhibitor for the prevention of HIV as pre-exposure prophylaxis (PrEP).
⬥︎ In June 2025, Cidara Therapeutics, Inc., a biotechnology company applying its proprietary Cloudbreak platform to develop drug-Fc conjugate (DFC) therapeutics, announced positive topline results from its randomized, double-blind, placebo-controlled Phase 2b NAVIGATE trial evaluating CD388 for the prevention of seasonal influenza in healthy unvaccinated adults aged 18 to 64.
⬥︎ In June 2025, Buscar Company announced the acquisition of a 70% stake in Armorgenix Company through a strategic stock swap. This transformative acquisition marks a significant expansion of Buscar's portfolio into the pharmaceutical sector, complementing its established operations in natural resources and sustainable technologies.
You can place an order or ask any questions, please feel free to contact at sales@novaoneadvisor.com | +1 804 441 9344
Related Report –
⬥︎ Myelodysplastic Syndrome Drugs Market - https://www.novaoneadvisor.com/report/myelodysplastic-syndrome-drugs-market-
⬥︎ U.S. Antifungal Drugs Market - https://www.novaoneadvisor.com/report/us-antifungal-drugs-market
⬥︎ Therapeutic Drug Monitoring Market- https://www.novaoneadvisor.com/report/therapeutic-drug-monitoring-market
⬥︎ General Anesthesia Drugs Market - https://www.novaoneadvisor.com/report/general-anesthesia-drugs-market
⬥︎ LAMEA Oncology/Anti-cancer Drugs Market - https://www.novaoneadvisor.com/report/lamea-oncology-anti-cancer-drugs-market
⬥︎ Oncology Drugs Market - https://www.novaoneadvisor.com/report/oncology-drugs-market
⬥︎ Asthma and COPD Drugs Market - https://www.novaoneadvisor.com/report/asthma-and-copd-drugs-market
Segments Covered in the Report
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc. has segmented the U.S. antiviral drugs market.
By Type
• Branded
• Generics
By Drug Class
• DNA Polymerase Inhibitors
• Reverse Transcriptase Inhibitors
• Protease Inhibitors
• Neuraminidase Inhibitors
• Others
By Application
• HIV
• Hepatitis
• Herpes
• Influenza
• Others
By Distribution Channel
• Hospital Pharmacy
• Retail Pharmacy
• Online Pharmacy
Immediate Delivery Available | Buy This Premium Research https://www.novaoneadvisor.com/report/checkout/9193
About-Us
Nova One Advisor is a global leader in market intelligence and strategic consulting, committed to delivering deep, data-driven insights that power innovation and transformation across industries. With a sharp focus on the evolving landscape of life sciences, we specialize in navigating the complexities of cell and gene therapy, drug development, and the oncology market, enabling our clients to lead in some of the most revolutionary and high-impact areas of healthcare.
Our expertise spans the entire biotech and pharmaceutical value chain, empowering startups, global enterprises, investors, and research institutions that are pioneering the next generation of therapies in regenerative medicine, oncology, and precision medicine.
Web: https://www.novaoneadvisor.com/
Contact Us
USA: +1 804 420 9370
Email: sales@novaoneadvisor.com
For Latest Update Follow Us: LinkedIn

