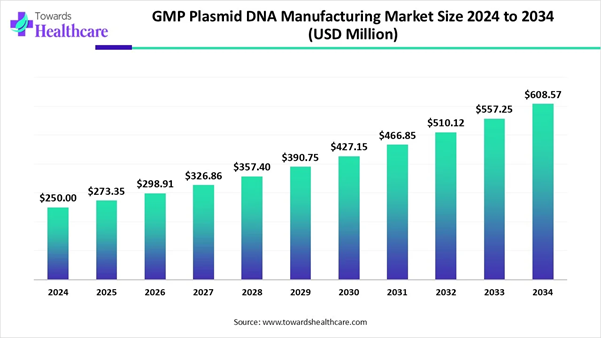
The global GMP (Good Manufacturing Practice) plasmid DNA manufacturing market size was valued at around USD 250 million in 2024. It is expected to grow to USD 273.35 million in 2025 and is projected to reach approximately USD 608.57 million by 2034. This reflects a strong growth trend, with the market expanding at a CAGR of 9.34% between 2025 and 2034.
Broadly influencing factors in market growth are increased use of plasmid DNA in cell and gene therapies, rising demand for DNA vaccine development in clinical trials, and commercialization. Also, the escalating regulatory standards for the quality of plasmids employed in biomanufacturing, boosting advances in therapeutic modalities such as CAR-T cell therapies and gene editing technologies, are playing a major role in the expansion of the GMP plasmid DNA manufacturing market.
Get a sneak peek into key market trends and data with our sample report @ https://www.towardshealthcare.com/download-sample/5638
GMP Plasmid DNA Manufacturing Market Highlights
• North America dominated the market in 2024.
• Asia Pacific is expected to be the fastest-growing region in the upcoming years.
• By product, the plasmid DNA segment held the major revenue share of the market in 2024.
• By product, the viral vectors segment is expected to grow significantly during the forecast period.
• By type, the preclinical therapeutics segment led the market in 2024.
• By type, the clinical therapeutics segment is expected to grow at the fastest CAGR in the projected period.
• By application, the DNA vaccines segment dominated in the GMP plasmid DNA manufacturing market in 2024.
• By application, the gene therapy segment is expected to grow fastest over the projected timeframe.
Access detailed market figures, growth forecasts, and regional insights in our comprehensive databook @ https://www.towardshealthcare.com/download-databook/5638
Market Overview
The GMP plasmid DNA manufacturing is a process in which plasmid DNA is manufactured in stringent guidelines and quality standards, emphasizing the regulatory considerations that meet for application in clinical trials, biopharmaceutical production, and other uses. Nowadays, in various sectors, they are applied, such as in gene therapy for the treatment of different conditions, vaccine developments, and most probably in immunotherapy to target and eliminate cancer cells or fight against cancer.
Robust Acquisition in Vaccine Production and Immunotherapy: Major Potential
GMP plasmid DNA manufacturing market, expanded in the high-quality production of vaccines, which involves a process from starting plasmid design to large-scale production, following regulatory standards. Different types of vaccines example, are ZyCoV-D (Cadila Healthcare), pVAC (DNA vaccine plasmid), Smallpox DNA Vaccine, and DNA vaccines for other diseases, including vaccines for malaria, AIDS, influenza, Ebola, and herpesvirus. However, in immunotherapy, GMP plasmid DNA manufacturing is widely used in viral vector production, mRNA production, and in CAR-T cell therapy, etc.
GMP Plasmid DNA Manufacturing Market: Regional Analysis
North America held the dominating share of the market in 2024. Due to the major factors such as breakthroughs in cell and gene therapies used in numerous genetic conditions, along with rapid development in cancer treatment therapies, especially for cancer-specific gene therapies. Moreover, the expansion of the market is highlighted by robust research and development infrastructure in North America.
The significantly dominating country in North America is the US, experiencing the adoption of novel and advanced, automated technologies in GMP plasmid DNA manufacturing to improve plasmid DNA production effectiveness and countability; also, both global and new industries are investing in the expansion of the manufacturing capabilities.
For instance,
• In April 2025, ProBio, a global CDMO, launched GMP plasmid DNA manufacturing services in Hopewell.
Additionally, Canada is the one country that has been facing an enormous growth in the GMP plasmid DNA manufacturing market, due to the increasing demand for gene therapy, in which plasmid DNA is a vital portion, along with the accelerating investments in biotechnology for vaccine R&D and regulatory support are merging as the important drivers.
Get the latest insights on healthcare industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership
The Asia Pacific is Anticipated to Show the Fastest Growth in the Projected Period
In ASAP, the expansion of the GMP plasmid DNA manufacturing market, experiencing due to the accelerating investments in biotechnology and pharmaceuticals, boosting healthcare needs, and a major focus on gene therapies and regenerative medications. Moreover, a growing existence of untapped opportunities, commercial development, optimizing healthcare infrastructure, and encouraging government initiatives are highly impacting the respective market. The global emerging countries in ASAP are China, India, and Japan, by produce high-quality products with less expense in production.
For instance,
• In March 2025, China-based WuXi Biologics, a leading global Contract Research, Development, and Manufacturing Organization (CRDMO), launched a new platform to accelerate the production of recombinant protein and plasmid DNA.
In India, a growing surge in clinical developments, including cell and DNA therapies, DNA vaccines, and increasing regulatory initiatives in biotechnology, is expected with the expansion of the biopharmaceutical field, which is enhancing the contribution to market growth.
For instance,
• In February 2025, New York-based VJ Group announced about launch of its new journey, VJ Bio, a biotech company devoted to generating an integrated ecosystem for pioneers to modify their advances into affordable and effective next-generation therapies.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
The GMP Plasmid DNA Manufacturing Market Segmentation Analysis
By product type analysis
The plasmid DNA segment led the market in 2024. Contributing significant factors are increasing demand in gene therapies, raised investments in biotechnology, developments in synthetic biology, and the growth of the biopharmaceutical field. Also, ensuring robust bacterial growth and replication in cells is crucial for the production of huge amounts of plasmid in clinical applications.
On the other hand, the viral vectors segment is expected to grow significantly in the upcoming years. This segment is driven by the application in gene and cell therapy in various clinical trials and successful approvals for Zolgensma and CAR-T therapies. Besides this, advancements in viral vector engineering, including the development of novel vector types and optimized delivery systems, are providing a highly efficient and accurate gene therapy process.
By type analysis
By type, the preclinical therapeutics segment held the major revenue share of the market in 2024. Primarily, the GMP-grade plasmid DNA is required in preclinical studies, particularly in animal testing for drug safety, efficacy, and metabolism. Moreover, the development of mRNA vaccines and the rising approach in mRNA-based treatments for different conditions have raised a vital demand for high-quality plasmid DNA.
The clinical therapeutics segment is expected to be the fastest-growing segment in the projected period. The globally rising chronic diseases cases such as cancer, cardiovascular conditions, and genetic disorders, are demanding innovative therapeutics, including gene therapy, vaccines. As well as, merging developments in cell and gene therapies are furthermore impelling the demand for GMP-grade plasmid DNA in the ongoing clinical trials or in R&D innovations.
By application analysis
The DNA vaccines segment led the GMP plasmid DNA manufacturing market in 2024. As escalating technical innovations in genetic engineering, fermentation, and plasmid purification techniques are enabling the improved efficiency, extensibility, and reasonable cost of plasmid DNA production, making it a great practical and workable choice for vaccine development. As well, moving towards the mRNA production is boosting the segment expansion.
On the other hand, the gene therapy segment is expected to grow fastest over the projected timeframe. The GMP plasmid DNA manufacturing market, by this segment facing crucial growth, impelled by the ongoing research activities targeted on the development of highly efficient and safe gene therapies, which need high-quality plasmid DNA for R&D. Although, the FDA has approving gene therapies for particular conditions, including advanced melanoma and chronic lymphocytic leukemia, which is boosting prospect confidence of the market.
Elevate your healthcare strategy with Towards Healthcare. Enhance efficiency and drive better outcomes schedule a call today: https://www.towardshealthcare.com/schedule-meeting

GMP Plasmid DNA Manufacturing Market Companies:
• Thermo Fisher Scientific
• Cobra Bio
• Rescript Probe
• Wuxi Advanced Therapies
• Charles River
• Weisman Biomanufacturing
• Bioscience
• Lake Pharma
• Alderton
• Kaneka Neurogenetic S.A.
• Catalent Biologics
• Neurogenetic
• Nature Technology Corporation
• Vector Builder
• VGXI, Inc.
• Plasmid Factory
• Delphi Genetics
• Esko Aster
• Bionian
• Creative Biolabs
• Vilene Biosciences
• Patheon
• Cognate Bio Services
Browse More Insights of Towards Healthcare:
• The global DNA read, write and edit market is valued at $7.35 billion in 2024, expected to grow to $8.35 billion in 2025, and is projected to reach $26.46 billion by 2034, growing at a steady annual rate of 13.63%.
• The global viral vector and plasmid DNA manufacturing market was worth $6.01 billion in 2023 and is forecasted to grow rapidly to $43.04 billion by 2034, with an annual growth rate of 20.7%.
• The Asia-Pacific viral vector and plasmid DNA manufacturing market is estimated at $1.38 billion in 2024, rising to $1.68 billion in 2025, and is expected to reach $10.01 billion by 2034, growing at a strong rate of 21.93% per year.
• The DNA diagnostics market was valued at $10.69 billion in 2023 and is projected to reach $17.44 billion by 2034, growing at a moderate pace of 4.55% annually.
• The DNA sequencing market was worth $12.42 billion in 2023 and is expected to grow significantly to $107.63 billion by 2034, expanding at a fast rate of 21.69% each year.
• The global plasmid DNA manufacturing market stood at $1.85 billion in 2023 and is expected to reach $12.27 billion by 2034, growing at a yearly rate of 18.77%.
• The plasmid purification market is valued at $1.95 billion in 2024, expected to grow to $2.18 billion in 2025, and projected to reach $5.88 billion by 2034, growing at an annual rate of 11.64%.
• The cell therapy media market is worth $1.34 billion in 2024, rising to $1.49 billion in 2025, and is likely to reach $3.83 billion by 2034, with a growth rate of 11.14% per year.
• The cell sorting market is estimated at $293.33 million in 2024, growing to $318.67 million in 2025, and expected to hit $673.14 million by 2034, expanding at 8.64% annually.
• The single cell analysis market is valued at $5.19 billion in 2024, projected to grow to $6.16 billion in 2025, and expected to reach $29.15 billion by 2034, growing at a strong annual rate of 18.74%.
What is Going Around the Globe?
• In May 2025, GenScript, a leading player in life science research tools and services support, launched a GMP-like mRNA manufacturing service, created particularly to support preclinical studies and IND-enabling research.
• In February 2025, Novartis, a Swiss MNC in pharmaceuticals, inaugurated its foremost specialized viral vector production facility in Slovenia, Europe.
• In November 2024, NewBiologix, a technology innovation company developing tools, introduced its Xcell™ rAAV Production and Analytics Platform (Xcell rAAV Platform), created to allow gene therapy companies to identify and manufacture optimal rAAV candidates for preclinical and clinical studies.
• In September 2024, Aurora Biosynthetics, an innovative RNA therapeutics manufacturer, launched a set of novel RNA therapies in the ASAP region.
GMP Plasmid DNA Manufacturing Market Segmentation
By Product
• Plasmid DNA
• Viral Vectors
• Retroviral
• Adenoviral
• Lentiviral
• Adeno-Associated
• Others
• Non-viral
• Lipid/polymer
• Electroporation
• Nanoparticles
• Others
By Type
• Pre-Clinical Therapeutics
• Clinical Therapeutics
• Marketed Therapeutics
By Application
• DNA Vaccines
• Gene Therapy
• Immunotherapy
• Others
By Region
• North America
• U.S.
• Canada
• Asia Pacific
• China
• Japan
• India
• South Korea
• Thailand
• Europe
• Germany
• UK
• France
• Italy
• Spain
• Sweden
• Denmark
• Norway
• Latin America
• Brazil
• Mexico
• Argentina
• Middle East and Africa (MEA)
• South Africa
• UAE
• Saudi Arabia
• Kuwait
Unlock the full report for in-depth analysis, strategic insights, and future outlook on the GMP plasmid DNA manufacturing market @ https://www.towardshealthcare.com/price/5638
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Gain access to the latest insights and statistics in the healthcare industry by subscribing to our Annual Membership. Stay updated on healthcare industry segmentation with detailed reports, market trends, and expert analysis tailored to your needs. Stay ahead of the curve with valuable resources and strategic recommendations. Join today to unlock a wealth of knowledge and opportunities in the dynamic world of healthcare: Get a Subscription
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics, with a strong emphasis on life science research. Dedicated to advancing innovation in the life sciences sector, we build strategic partnerships that generate actionable insights and transformative breakthroughs. As a global strategy consulting firm, we empower life science leaders to gain a competitive edge, drive research excellence, and accelerate sustainable growth.
Our Trusted Data Partners
Precedence Research | Statifacts | Towards Packaging | Towards Automotive | Towards Food and Beverages | Towards Chemical and Materials | Towards Consumer Goods | Towards Dental | Towards EV Solutions | Nova One Advisor | Healthcare Webwire | Packaging Webwire | Automotive Webwire
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare

