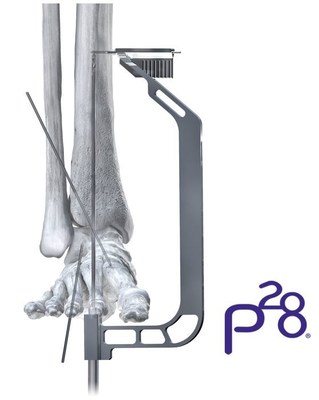
The Phantom® ActivCore Nail addresses TTC arthrodesis and is the second of three hindfoot fusion nailing systems Paragon 28® plans to launch in 2020.
|
ENGLEWOOD, Colo., Feb. 7, 2020 /PRNewswire/ -- The Phantom® ActivCore Nail addresses TTC arthrodesis and is the second of three hindfoot fusion nailing systems Paragon 28® plans to launch in 2020. The ActivCore Nail was designed to include an outer spring sheath and inner sliding core which allows for consistent active compression and up to 8 mm of bone resorption across all joint spaces. The Nail accommodates two 7.2 mm calcaneal pegs that are inserted from posterior to anterior at a variable (0-18°) angle. These pegs provide fixation distally and work with the inner core feature and tibial pegs to allow for active compression to pass through all joint surfaces.
The system leverages PRECISION® Guide technology allowing for reproducibility when placing a 3.0 mm drill-pin from distal to proximal, through the center of the calcaneus and up to the tibia. As with the previous Phantom® Hindfoot Nail System, all ActivCore Nails were engineered with a flex coil design in the proximal end to provide flexibility as the implant finds its way through the contouring of the tibial canal. All pegs are inserted from posterior to anterior and medial to lateral eliminating the need to take down the fibula. The Phantom® ActivCore Nails are constructed from Type II Anodized Titanium Alloy and will be offered in 22 size options with three diameters and four lengths. All distal diameters of the Nails are 13.3 mm in diameter with a transition to either; 10.0, 11.5 or 13.0 mm proximally. The distal diameter was increased at the joint spaces to add strength to the implant where it's needed most. As has been the case with previous hindfoot systems, the Phantom® ActivCore System will include a comprehensive offering of procedural instrumentation to address joint preparation and restoration of anatomic alignment. The ActivCore Nail is implanted utilizing the carbon fiber Phantom® Ghost™ Outrigger. The radiolucent and inlay features of the Outrigger allow for visualization of peg trajectory under fluoroscopy prior to drilling. Paragon 28® is planning for full commercial launch of the Phantom® ActivCore Nail System in Q2 2020. About Paragon 28® Working relentlessly to advance the science behind Foot and Ankle surgery, Paragon 28® is passionate about blending surgical philosophies from global thought leaders to develop biomechanically and clinically relevant solutions. We are committed to surgeon-centric systems, specialty instruments, and next generation implants at today's prices. For more information visit www.paragon28.com Contact: Jim Edson, jedson@paragon28.com
SOURCE Paragon 28, Inc. |