Paige today announced it has published the results of a pivotal study demonstrating accuracy gains in pathologists’ diagnosis of prostate cancer when using Paige Prostate Detect, an FDA-authorized AI software that assists pathologists in the detection of foci that are suspicious for cancer during the review of scanned whole slide images (WSI) from prostate needle biopsies.
Results published in Archives of Pathology & Laboratory Medicine served as foundation of first FDA authorized AI software in digital pathology
NEW YORK--(BUSINESS WIRE)-- Paige, a global leader in end-to-end digital pathology solutions and clinical AI applications, today announced it has published the results of a pivotal study demonstrating accuracy gains in pathologists’ diagnosis of prostate cancer when using Paige Prostate Detect, an FDA-authorized AI software that assists pathologists in the detection of foci that are suspicious for cancer during the review of scanned whole slide images (WSI) from prostate needle biopsies. The results were published in the Archives of Pathology & Laboratory Medicine and are available online here.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221221005169/en/
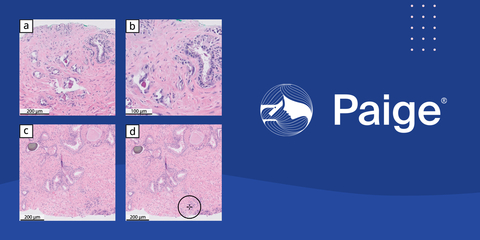
On a whole slide image with an ISUP Group 4 cancer involving 2% of tissue (c), Paige Prostate Detect indicates a focus of interest that is most likely to harbor cancerous tissue (d). The tissue was misclassified as benign by 12 pathologists and deferred by one pathologist. A benign seminal vesicle was misclassified as cancer by five pathologists and deferred by three in the study (a) (b), while Paige Prostate Detect correctly classified the image as benign. Photo: Paige
The study was designed to compare the diagnostic accuracy of pathologists reviewing images of prostate biopsies for the presence of prostate cancer with and without the assistance of Paige Prostate Detect. In the study, pathologists were shown to increase approximately eight percentage points in sensitivity in correctly diagnosing cancer (from 88.7% to 96.6%) with an increase in specificity as well (from 97.3% to 98%). This represents a 70% reduction in false negative diagnoses and a 24% reduction in false positive diagnoses.
Both non-specialist pathologists and specialist pathologists saw statistically significant sensitivity gains, with non-specialist pathologists showing a sensitivity of detection with assistance of Paige Prostate Detect similar to that of specialist pathologists. There was no statistical significance between improvements of pathologists who reviewed slides on-site or remotely, and there were no significant differences in performance across patient age, race and ethnicity.
“These findings show Paige Prostate Detect is a robust AI software that can be used broadly in clinical practice to provide information physicians need to guide the accurate diagnosis of prostate cancer,” said David Klimstra, M.D., Founder and Chief Medical Officer at Paige. “While specialist pathologists are critical, they may not be available at every hospital and laboratory. By providing non-specialist pathologists with Paige Prostate Detect, we are able to give them a user-friendly tool that serves as a second pair of eyes to improve confidence in the diagnostic process. In turn, this heralds a revolution in how pathology is practiced and reinforces the central role pathologists play in patient care.”
Paige Prostate Detect was tested on a wide range of tissue samples to simulate the cases pathologists often encounter in routine clinical practice. The dataset included 610 de-identified prostate needle biopsy whole slide images stained with H&E from 218 different institutions worldwide. Additionally, the data included a mix of hard and easy cases and was enriched with small tumor foci to challenge the system with lesions that can be overlooked by pathologists.
“The study provides an important validation of our underlying technology and of the first-ever AI software for digital pathology to be authorized by the FDA,” said Thomas Fuchs, Dr.Sc., Founder and Chief Scientist at Paige. “Paige Prostate Detect demonstrated robust performance on a challenging dataset. Furthermore, our analysis suggests there is no unknown bias in how Paige Prostate Detect works, a critical step in ensuring that such tools do not contribute to health care disparities. Together with other studies on Paige’s technology, this study again demonstrates that Paige Prostate Detect is generalizable to multiple laboratories around the world.”
About Paige
Paige is using the power of AI to drive a new era of cancer discovery and treatment. To improve the lives of patients with cancer, Paige has created a cloud-based platform that transforms pathologists’ workflow and increases diagnostic confidence as well as productivity, all on a global scale. Paige is the first company to receive FDA approval for a clinical AI application in digital pathology. The same Paige technology empowers pharmaceutical companies to more effectively evaluate treatment options for patients and design new biomarkers for drug development so that every patient gets precise treatment options.
For additional information, please visit: https://www.paige.ai, Twitter and LinkedIn.
View source version on businesswire.com: https://www.businesswire.com/news/home/20221221005169/en/
Source: Paige
Smart Multimedia Gallery
On a whole slide image with an ISUP Group 4 cancer involving 2% of tissue (c), Paige Prostate Detect indicates a focus of interest that is most likely to harbor cancerous tissue (d). The tissue was misclassified as benign by 12 pathologists and deferred by one pathologist. A benign seminal vesicle was misclassified as cancer by five pathologists and deferred by three in the study (a) (b), while Paige Prostate Detect correctly classified the image as benign. Photo: Paige







