New Jersey
Looking for an IT job? From data engineer to information security, check out the BioSpace list of 10 companies hiring life sciences professionals like you.
Looking for a research and development job? Check out the BioSpace list of 12 companies hiring life sciences professionals like you.
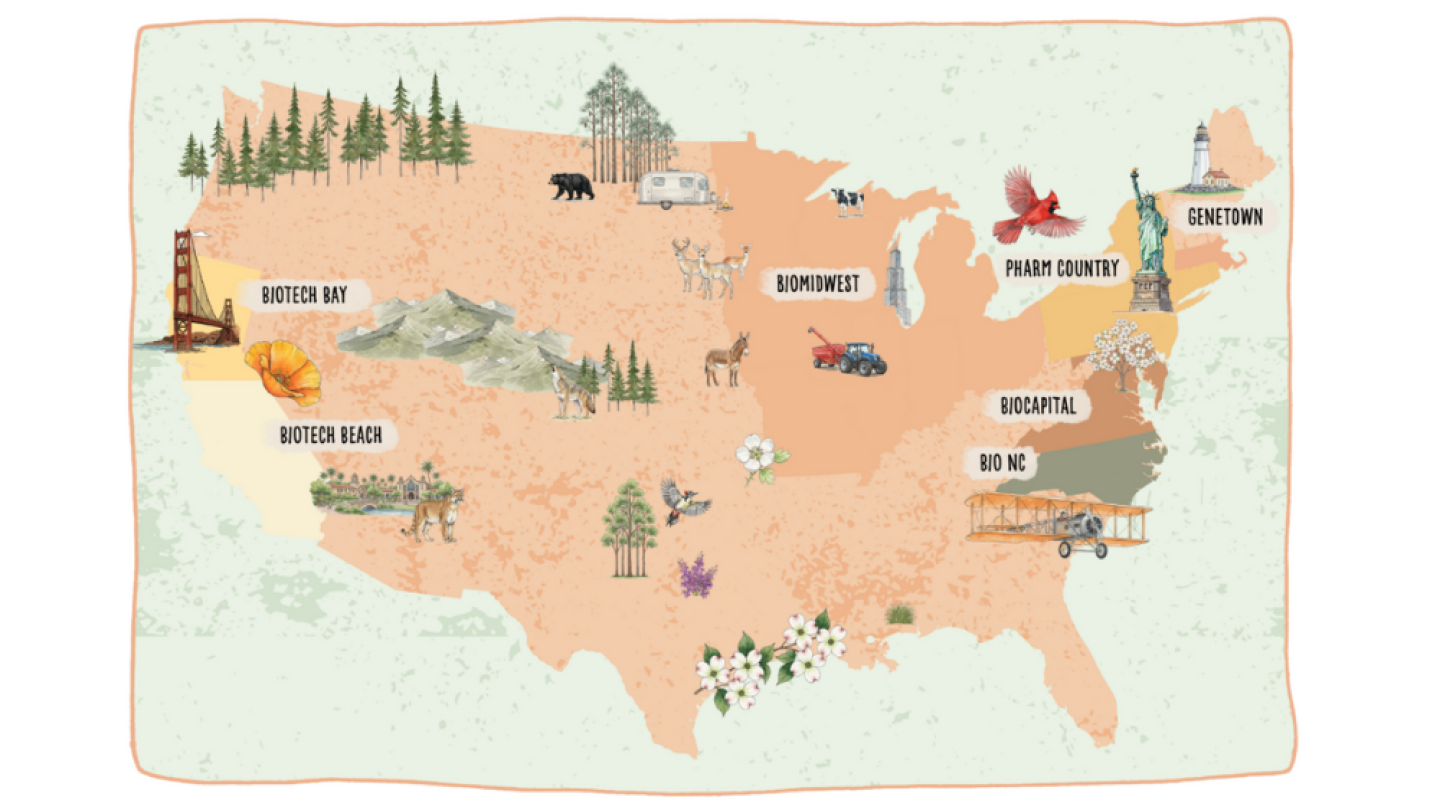
BioSpace has revealed its new 2026 Hotbed Maps, showcasing seven regional life sciences hot spots.
Looking for a manufacturing job? Check out the BioSpace list of 11 companies hiring life sciences professionals like you.
In 2025, made or projected biopharma workforce cuts affected about 42,700 employees, according to BioSpace tallies. BioSpace takes a deep dive into which companies and locations were impacted and speaks to experts about what to expect ahead—and why.
Looking for a new opportunity in New Jersey? These nine companies have open roles that could be a great fit for you.
BioSpace has named 50 life sciences companies to its 2026 Best Places to Work list. AbbVie, Amneal Pharmaceuticals and BridgeBio executives share what makes their organizations special.
Looking for a job in regulatory? Check out the BioSpace list of eight companies hiring life sciences professionals like you.
Due largely to CSL, Merck and Novo Nordisk’s reorganizations that could total about 19,350 people, Q3 cuts rose significantly year over year and quarter over quarter, based on BioSpace tallies.
Looking for a job in oncology? Check out the BioSpace list of nine companies hiring life sciences professionals like you.
PRESS RELEASES