MIMEDX Group, Inc. (NASDAQ: MDXG) (“MIMEDX” or the “Company”), an industry leader in utilizing amniotic tissue as a platform for regenerative medicine, today urged shareholders to consider several key questions about Prescience Point’s interests, motivations and actions regarding the disruptive proxy fight it is waging to gain undue influence over the MIMEDX Board of Directors
MARIETTA, Ga., May 04, 2021 (GLOBE NEWSWIRE) -- MIMEDX Group, Inc. (NASDAQ: MDXG) (“MIMEDX” or the “Company”), an industry leader in utilizing amniotic tissue as a platform for regenerative medicine, today urged shareholders to consider several key questions about Prescience Point’s interests, motivations and actions regarding the disruptive proxy fight it is waging to gain undue influence over the MIMEDX Board of Directors. MIMEDX believes shareholders deserve to have the facts before voting their shares at the Company’s 2021 Annual Meeting of Shareholders (“Annual Meeting”).
Before voting their shares, MIMEDX shareholders should consider what motivations truly drive Prescience Point’s campaign.
THE QUESTIONS:
1. Is it ethical or appropriate to buy shares, publish a promotional “report” hyping a nearly $32 price target, and quickly sell shares for a profit when the stock price rises after the “report” is disseminated?
- Prescience Point bought shares before it issued its December 2020 “report,” 1 then quickly flipped an equivalent amount of shares following the stock rise that occurred after issuing its “report.”
2. Is Prescience Point acting on behalf of all MIMEDX shareholders or only its own interests?
3. Why is Prescience Point seeking to replace its OWN director nominees, appointed just two years ago -- Board Chair, Dr. M. Kathleen Behrens and Audit Committee Chair, K. Todd Newton – both of whom Prescience Point itself said add value to the Board in view of their “immense credibility and reputational capital”, are “world-class” and would “bring value to all shareholders”?2 Why does Prescience Point now seek to oust its OWN director nominees who have been outstanding leaders on the Board?
4. What is Prescience Point’s shelf life for director designees? Less than two years?
5. Why has Prescience Point nominated candidates with no or very little public company board experience?
6. Why did Prescience Point turn down our settlement offer that addressed its demand for board representation and additional investment by the Company in our shareholder communications program?
7. Is Prescience Point actually seeking control when it seeks to replace the board seats of key board leaders – Chair, CEO, and Audit Committee Chair?
8. Is Prescience Point just pushing for a fire-sale of the Company because their fund is so heavily invested in MIMEDX?
- Prescience Point’s portfolio is extremely concentrated in MDXG – more than 73% of its estimated assets under management (AUM) as of December 31, 2020 are invested in MDXG.
9. What are Prescience Point’s motivations behind pursuing a quick sale, at this stage in the Company’s clinical trial pipeline?
- It is more likely shareholders capture substantially greater value through a transaction after a company has clinical data results in a U.S. Food & Drug Administration (FDA) approved treatment for indications which command a sizeable market with commercialization plans underway.
10. Why would Prescience Point push a company to take overly-aggressive positions regarding our products that may be counter to FDA best practices – especially now, when the Company has finally restored its credibility with both the FDA and Securities and Exchange Commission (SEC)?
THE FACTS
Publicly reported trading activity shows that Prescience Point has generated short-term trading profits driven by its promotional, self-publication, of a “report” with lofty share price targets:
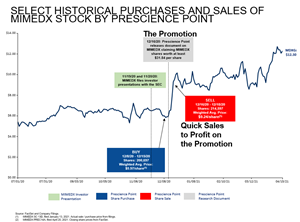
- Prescience Point purchased approximately 200,697 shares of MIMEDX common stock between December 08, 2020 and December 10, 2020.3 They admit this in their January 13, 2021 Schedule 13D filing with the SEC.
- On December 16, 2020, Prescience Point authored and self-published an opinion piece4 stylized to resemble the professional, digital packaging of a sell-side analyst report issued by a prominent Wall Street firm. In their “report”, Prescience Point commends MIMEDX’s turnaround success, leadership team and the “blockbuster, game-changing” potential of AMNIOFIX for the treatment of knee osteoarthritis, disclosing a stock price target of nearly $32.
- MIMEDX’s share price at the time of the Prescience Point promotional “report” was $6.58.
- Within two days of releasing its “report,” Prescience Point opportunistically sold 214,597 shares of MIMEDX’s common stock, at prices ranging from $8.12 to $9.61, despite having a target price of nearly $32. These price points represent a premium range of 23% to 46% over the Company’s closing stock price on the trading day immediately prior to the self-publication of Prescience Point’s “report.” (See chart. https://www.globenewswire.com/NewsRoom/AttachmentNg/22300c39-ca08-4222-91df-569e53ae8c30)
In its Proxy statement, Prescience Point takes credit for the stock price increase following the publication of its promotional “report” but neglects to disclose its subsequent stock sales at a higher price. 5
Do not allow Prescience Point to put your investment at risk by giving them further and undeserved influence over the Board and therefore, the business of MIMEDX. Prescience Point’s demands and commentary indicate that they do not understand responsible or compliant public pharmaceutical company disclosure, and instead are advocating for speculative actions designed to satisfy their own agenda.
The MIMEDX Board and management team have the opportunity to continue the successful execution of the Company’s clear strategic plan to accelerate its late-stage pipeline, achieve its stated top-line growth objectives in its core business and drive shareholder value.
The Company’s Board of Directors and management team have taken decisive and positive actions to create shareholder value by restoring the Company’s integrity, improving business liquidity, and transforming the culture of the organization. MIMEDX stock has appreciated 237% since the Company appointed Timothy R. Wright as CEO in May of 2019.
Today, MIMEDX is a much stronger company that is very well-positioned to capitalize on the growing opportunities in the regenerative medicine industry.
Our transformation is not complete. MIMEDX’s future success is dependent on continuing to execute and operate our business in a compliant and transparent manner. Any action that puts this approach at risk puts the Company’s progress, and the future of its shareholders’ investment, at risk.
The MIMEDX Board of Directors unanimously recommends that shareholders vote the WHITE proxy card FOR MIMEDX’s four highly qualified directors standing for election - Dr. M. Kathleen Behrens, Mr. Todd Newton, Mr. Timothy R. Wright, and Dr. Phyllis Gardner.
PROTECT YOUR INVESTMENT! VOTE THE WHITE PROXY CARD TODAY “FOR ALL” FOUR OF MIMEDX’S HIGHLY QUALIFIED DIRECTOR NOMINEES
| Your Vote Is Important, No Matter How Many |
| or How Few Shares You Own |
| You can vote by Internet, telephone or by signing and dating the WHITE proxy card and mailing it in the envelope provided. |
| If you have any questions about how to vote your shares, or need additional assistance, please contact: |
| MORROW |
| SODALI |
| MDXG@investor.Morrowsodali.com |
| (203) 658-9400 |
| or |
| Toll-Free (800) 662-5200 |
MIMEDX will be holding its Annual Meeting virtually on May 27, 2021 at 10:00 a.m. Eastern Time at www.cesonlineservices.com/mdxg21_vm. MIMEDX shareholders of record as of 5:00 p.m. Eastern Time on April 16, 2021 are entitled to vote at the Annual Meeting.
MIMEDX’s definitive proxy materials, letter to shareholders and other relevant information can be found at https://votemimedx.com/.
Important Cautionary Statement
This communication contains forward-looking statements, including, among other things, statements regarding: (i) our strategic plan, as illustrated by our current business priorities, and our ability to implement these priorities; (ii) our expectations regarding the sufficiency of our liquidity and existing capital resources to implement our current business priorities; (iii) the advantages of our products and development of new products; (iv) our expectation regarding the size of the potential market and any growth in such market; (v) the likelihood, timing, and scope of possible regulatory approval and commercial launch of our late-stage product candidates and new indications for our products; (vi) the status, timing, and expected results of the Company’s clinical trials and planned regulatory submissions, and our expectations regarding our ability to potentially accelerate the timing of any trial or regulatory submission; (vii) our ability to transform the Company’s culture while maintaining its integrity; (viii) the effectiveness of amniotic tissue as a therapy for any particular indication or condition; (ix) estimates of potential addressable markets for our potential future products; and (x) our expectations regarding the effects of the proxy contest launched by Prescience Point. Additional forward-looking statements may be identified by words such as “believe,” “expect,” “may,” “plan,” “goal,” “outlook,” “potential,” “will,” “preliminary,” and similar expressions, and are based on management’s current beliefs and expectations.
Forward-looking statements are subject to risks and uncertainties, and the Company cautions investors against placing undue reliance on such statements. Actual results may differ materially from those set forth in the forward-looking statements. Factors that could cause actual results to differ from expectations include: (i) notwithstanding the FDA’s statement on April 21, 2021, there remain a number of uncertainties regarding the application of the FDA’s regulations to the Company’s products and practices, and the Company may adjust its plans to comply with FDA’s requirements; (ii) there can be no assurance that the FDA will further extend enforcement discretion to cover products that have a regulatory approval pending, nor can there be any assurance that the Company will even be able to engage with the FDA on the subject; (iii) the Company’s estimate of the impact of enforcement discretion assumes that the Company is able to sell its products through May 31, 2021, and that the Company may continue to sell its cord products thereafter; (iv) the status, timing, and expected results of the Company’s clinical trials and planned regulatory submissions, and our expectations regarding our ability to potentially accelerate the timing of any trial or regulatory submission depend on a number of factors including favorable trial results, patient access, and our ability to manufacture in accordance with CGMP and appropriate chemistry and manufacturing controls; (v) the Company may change its plans due to unforeseen circumstances, and delay or alter the timeline for future trials, analyses, or public announcements; (vi) generally any meeting with the FDA depends on successful clinical trial results and the availability of such a meeting and its timing is outside of the Company’s control; (vii) the results of a clinical trial or trials may have little or no statistical value, or may fail to demonstrate that the product is safe or effective; (viii) our estimates of potential addressable markets for our potential future products are merely estimates and will depend on market acceptance of our potential, future products; (ix) we depend on our senior leadership team and may not be able to retain or replace these employees or recruit additional qualified personnel; and (x) actions that Prescience Point may take in connection with the proxy contest, which are outside our control. The Company describes additional risks and uncertainties in the Risk Factors section of its most recent annual report and quarterly reports filed with the SEC. Any forward-looking statements speak only as of the date of this communication and the Company assumes no obligation to update any forward-looking statement.
Important Information
The Company, its directors, director nominees and certain of its executive officers are participants in the solicitation of proxies from shareholders in respect of the Annual Meeting. The Company has filed a definitive proxy statement and associated WHITE proxy card in connection with the solicitation of proxies for the Annual Meeting with the SEC. Details concerning the nominees of the Company’s board of directors for election at the Annual Meeting are set forth in the definitive proxy statement. BEFORE MAKING ANY VOTING DECISION, SHAREHOLDERS OF THE COMPANY ARE URGED TO READ ALL RELEVANT DOCUMENTS FILED WITH OR FURNISHED TO THE SEC, INCLUDING THE COMPANY’S DEFINITIVE PROXY STATEMENT AND ANY SUPPLEMENTS THERETO, AS THEY CONTAIN IMPORTANT INFORMATION. Information regarding the identity of the Company’s participants and their respective interests in the matters to be voted on at the Annual Meeting, by security holdings or otherwise, are set forth in the definitive proxy statement and other documents filed with the SEC in connection with the Annual Meeting. Investors and shareholders can obtain a copy of the definitive proxy statement and other documents filed by the Company free of charge from the SEC’s website at www.sec.gov. The Company’s shareholders can also obtain, without charge, a copy of the definitive proxy statement and other relevant filed documents from the “SEC Filings” section of the Company’s website at www.mimedx.com.
About MIMEDX
MIMEDX is an industry leader in utilizing amniotic tissue as a platform for regenerative medicine, developing and distributing placental tissue allografts with patent-protected, proprietary processes for multiple sectors of healthcare. As a pioneer in placental biologics, we have both a core business, focused on addressing the needs of patients with acute and chronic non-healing wounds, and a promising late-stage pipeline targeted at decreasing pain and improving function for patients with degenerative musculoskeletal conditions. We derive our products from human placental tissues and process these tissues using our proprietary methods, including the PURION® process. We employ Current Good Tissue Practices, Current Good Manufacturing Practices, and terminal sterilization to produce our allografts. MIMEDX has supplied over two million allografts, through both direct and consignment shipments. For additional information, please visit www.mimedx.com.
Contacts:
Investors:
Jack Howarth
Investor Relations
404-360-5681
jhowarth@mimedx.com
Media:
Hilary Dixon
Corporate Communications
770-651-9307
hdixon@mimedx.com
1https://www.presciencepoint.com/wp-content/uploads/2020/12/MDXG-Amniofix-Report-FINAL.pdf
2 Prescience Schedule 13D/A, filed with the SEC on May 8, 2019
3https://www.sec.gov/Archives/edgar/data/0001765316/000101143821000032/form_sc13d-mimedx.htm
4https://www.presciencepoint.com/wp-content/uploads/2020/12/MDXG-Amniofix-Report-FINAL.pdf
5 Prescience Schedule 14A, page 4, filed with the SEC on May 03, 2021.