- nextBioPharmDSP Horizon 2020 project led by Lek Pharmaceuticals, a Sandoz company in Slovenia, highlighted as success storyfor research and innovation
- Completed 18-month milestone submission to EU
- MilliporeSigma provides expertise in continuous and flow-through downstream processing approaches and single-use systems
BILLERICA, Mass., Sept. 27, 2017 /PRNewswire/ -- MilliporeSigma today announced an update on its participation in Horizon 2020, the EU Framework Program for Research and Innovation, to improve biopharmaceutical downstream processing.
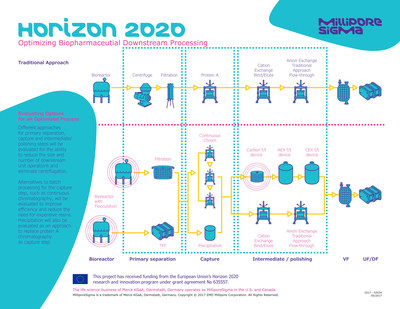
The nextBioPharmDSP, a consortium of seven public and private organizations, is developing a more efficient, cost-effective and environmentally friendly downstream process to manufacture monoclonal antibodies and biosimilars. Efforts focused on advanced analytical tools for process monitoring have already led to more publications and two patent applications, and additional publications and patent applications are being prepared in other areas of the project. The consortium has received approval for its 18-month periodical report, and the project was highlighted as a success story by the European Commission Directorate-General for Research and Innovation.
"The biopharmaceutical industry is facing pressure to reduce manufacturing costs and deliver greater efficiencies, all while being environmentally responsible," said Udit Batra, CEO, MilliporeSigma. "It is important for companies like MilliporeSigma and our customers to help address industry-wide challenges and accelerate the process of getting drugs to patients in need. Through the Horizon 2020 program, consortium members are already delivering important advances for downstream processing."
Collaboration outcomes will include reduction in the size and number of downstream unit operations as well as elimination of the need for centrifugation. Alternative approaches for the capture step are expected to improve efficiency and reduce the need for expensive resin volume. Capture via precipitation is also being studied as a replacement for protein A chromatography.
A disposable continuous chromatography system is in development with novel analytical tools and sensors, which are also implemented in other parts of downstream process. Single-use disposable technology for all downstream processing operations will be evaluated and flow-through approaches for polishing steps implemented, to remove impurities in a continuous way. Selected approaches will be integrated in a connected continuous downstream single-use process.
"Next generation bioprocessing strategies will improve our industry's ability to efficiently and cost-effectively supply high quality medicines," said Uro Urleb, Global Head Technical Development Biosimilars in the Biologics Technical Development and Manufacturing Unit at Novartis. "The consortium is delivering new approaches to the conventional manufacturing workflow and we expect the results of our efforts will help address variations in capacity needs, enable sustained drug supply and offer important environmental benefits."
The nextBioPharmDSP is part of the Horizon 2020 initiative, the largest EU-based program of its kind to date with nearly $96 billion of funding available over seven years (2014 to 2020). The program seeks to ensure Europe produces world-class science by removing barriers to innovation and collaboration between public and private sectors. It promises more breakthroughs, discoveries and world-firsts by taking great ideas from the lab to the market.
The project is coordinated by Lek Pharmaceuticals, a Sandoz company in Slovenia. Consortium members include Sandoz GmbH (Austria), University of Natural Resources and Life Sciences, Vienna (Austria), Karlsruhe Institute of Technology (Germany), National Institute of Chemistry (Slovenia) and National Systems srl (Italy). MilliporeSigma participates in the consortium through its French operating entity, Millipore S.A.S. For more information, please visit the project website at http://nextbiopharmdsp.eu/.
All Merck KGaA, Darmstadt, Germany news releases are distributed by email at the same time they become available on the EMD Group website. In case you are a resident of the U.S. or Canada please go to www.emdgroup.com/subscribe to register again for your online subscription of this service as our newly introduced geo-targeting requires new links in the email. You may later change your selection or discontinue this service.
About the Life Science Business of Merck KGaA, Darmstadt, Germany
The Life Science business of Merck KGaA, Darmstadt, Germany, which operates as MilliporeSigma in the U.S. and Canada, has 20,000 employees and 65 manufacturing sites worldwide, with a portfolio of more than 300,000 products enabling scientific discovery. Udit Batra is the global chief executive officer of MilliporeSigma.
Merck KGaA, Darmstadt, Germany completed its $17 billion acquisition of Sigma-Aldrich in November 2015, creating a leader in the $125 billion global life science industry.
Merck KGaA, Darmstadt, Germany is a leading company for innovative and top-quality high-tech products in healthcare, life science and performance materials. The company has six businesses Biopharmaceuticals, Consumer Health, Allergopharma, Biosimilars, Life Science and Performance Materials and generated sales of 15 billion in 2016. Around 50,000 employees work in 66 countries to improve the quality of life for patients, to foster the success of customers and to help meet global challenges.
Merck KGaA, Darmstadt, Germany is the world's oldest pharmaceutical and chemical company since 1668, the company has stood for innovation, business success and responsible entrepreneurship. Holding an approximately 70 percent interest, the founding family remains the majority owner of the company to this day. The company holds the global rights to the name and the trademark "Merck" internationally except for the United States and Canada, where the company operates as EMD Serono, MilliporeSigma and EMD Performance Materials.
Acknowledgement:
This project has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement No 635557.
View original content with multimedia:http://www.prnewswire.com/news-releases/milliporesigma-provides-update-on-eus-horizon-2020-program-to-improve-biopharmaceutical-downstream-processing-300526205.html
SOURCE MilliporeSigma





