GS1 US has published a new guideline, “Best Practice Guide for Sharing Vital Attributes in Healthcare,” to help healthcare trading partners share data about medical devices or pharmaceutical products.
|
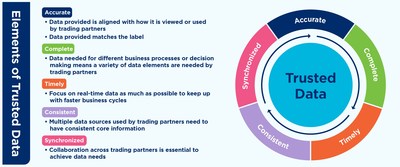
EWING, N.J., July 29, 2021 /PRNewswire/ -- GS1 US has published a new guideline, "Best Practice Guide for Sharing Vital Attributes in Healthcare," to help healthcare trading partners share data about medical devices or pharmaceutical products. The guideline is a resource for healthcare professionals charged with master data management, procurement, regulatory compliance and data governance. Developed by members of the GS1 Healthcare US Initiative in collaboration with the Healthcare Supply Chain Association (HSCA), the document offers best practices for the synchronization of product attributes such as definitions and formatting for brand name and product description. It includes recommendations for meeting five elements of "trusted data" – accurate, complete, timely, consistent and synchronized – which are needed to support efficient data exchange for basic healthcare supply chain processes such as product identification, order-to-cash and traceability. "When healthcare stakeholders collaborate using global data standards, their ability to trust and seamlessly synchronize shared data will improve," said Angela Fernandez, vice president of community engagement, GS1 US. "Alignment on attributes has a beneficial ripple effect, positively impacting data accuracy, efficiency and interoperability – all leading to improved healthcare operations and patient care." This guideline also covers data recommendations for the U.S. Food and Drug Administration (FDA) Drug Supply Chain Security Act (DSCSA) enabling pharmaceutical traceability and Unique Device Identification Rule (UDI) requirements for medical device traceability. For more information about GS1 US, the guideline and additional data quality healthcare resources, visit www.gs1us.org. About GS1 US:
SOURCE GS1 US |